Refer to NEET Chemistry Structure Of Atom MCQs Set A provided below available for download in Pdf. The MCQ Questions for Full Syllabus Chemistry with answers are aligned as per the latest syllabus and exam pattern suggested by NEET, NCERT and KVS. Structure Of Atom Full Syllabus MCQ are an important part of exams for Full Syllabus Chemistry and if practiced properly can help you to improve your understanding and get higher marks. Refer to more Chapter-wise MCQs for NEET Full Syllabus Chemistry and also download more latest study material for all subjects
MCQ for Full Syllabus Chemistry Structure Of Atom
Full Syllabus Chemistry students should refer to the following multiple-choice questions with answers for Structure Of Atom in Full Syllabus.
Structure Of Atom MCQ Questions Full Syllabus Chemistry with Answers
Question: An isotone of
Ge is
- a)
- b)
- c)
- d)
Answer:
Question: The ratio of specific charge of an electron to that of a proton is
- a) 1 : 1
- b) 1837 : 1
- c) 1 : 1837
- d) 2 : 1
Answer: 1837 : 1
Question: Atomic number and mass number of an element M are 25 and 52 respectively. The number of electrons, protons and neutrons in M2+ ion are respectively
- a) 25, 25 and 27
- b) 25, 27 and 25
- c) 27, 25 and 27
- d) 23, 25 and 27
Answer: 23, 25 and 27
Question: According to Bohr’s theory angular momentum of an electron in 6th orbit is
- a)
- b)
- c)
- d)
Answer:
Question: If r1 is the radius of the first orbit of hydrogen atom, then the radii of second, third and fourth orbits in term of r1 are
- a) r12, r13, r14
- b) 4r1 , 9r1 , 16r1
- c) 8r1, 27r1, 64r1
- d) 2r1, 6r1, 8r1
Answer: 4r1 , 9r1 , 16r1
Question: Electronic energy is negative because
- a) Electron has negative charge
- b) Energy is zero near the nucleus and decreases as the distance from nucleus increases
- c) Energy is zero at infinite distance from the nucleus and decreases as the electron comes towards nucleus
- d) These are interelectronic repulsions
Answer: Energy is zero at infinite distance from the nucleus and decreases as the electron comes towards nucleus
Question: An electron jumps from lower orbit to higher orbit, when
- a) Energy is released
- b) Energy is absorbed
- c) No change in energy
- d) It radiates energy
Answer: Energy is absorbed
Question: If the energy difference between the ground state and excited state of an atom is 4.4 × 10–19 J. The wavelength of photon required to produce this transition is
- a) 4.5 × 10–7 m
- b) 4.5 × 10–7 nm
- c) 4.5 × 10–7 Å
- d) 4.5 × 10–7 cm
Answer: 4.5 × 10–7 m
Question: The number of photons of light of wavelength 7000 Å equivalent to 1 J are
- a) 3.52 × 10–18
- b) 3.52 × 1018
- c) 50,000
- d) 10,0000
Answer: 3.52 × 1018
Question: The threshold energy is given as E0 and radiation of energy E falls on metal, then K.E. is given as
- a)
- b) E – E0
- c) E0– E
- d)
Answer: E – E0
Question: The frequency of a wave is 6 × 1015 s–1. Its wave number would be
- a) 105 cm–1
- b) 2 × 107 m–1
- c) 2 × 107 cm–1
- d) 2 × 105 cm–1
Answer: 2 × 107 m–1
Question: If threshold wavelength (λ°) for ejection of electron from metal is 330 nm, then work function for the photoelectric emission is
- a) 6 × 10–10 J
- b) 1.2 × 10–18 J
- c) 3 × 10–19 J
- d) 6 × 10–19 J
Answer: 6 × 10–19 J
Question: The ionization energy of the electron in the lowest orbit of hydrogen atom is 13.6 eV. The energies required in eV to remove electron from three lowest orbits of hydrogen atom are
- a) 13.6, 6.8, 8.4
- b) 13.6, 10.2, 3.4
- c) 13.6, 27.2, 40.8
- d) 13.6, 3.4, 1.51
Answer: 13.6, 3.4, 1.51
More Questions........................................................
Question: A certain metal when irradiated with light (v = 3.2 × 1016 Hz) emits photo electrons with twice kinetic energy as did photo electrons when the same metal is irradiated by light (v = 2.0 × 1016 Hz). Calculate v0 of electron?
- a) 1.2 × 1014 Hz
- b) 8 × 1015 Hz
- c) 1.2 × 1016 Hz
- d) 4 × 1012 Hz
Answer: 8 × 1015 Hz
Question: En = –313.6/n2 kcal/mole. If the value of E = –34.84 kcal/mole, to which value does ‘n’ correspond?
- a) 4
- b) 3
- c) 2
- d) 1
Answer: 3
Question: Which transition of Li2+ is associated with same energy change as n = 6 to n = 4 transition in He+?
- a) n = 3 to n = 1
- b) n = 8 to n = 6
- c) n = 9 to n = 6
- d) n = 2 to n = 1
Answer: n = 9 to n = 6
Question: Zeeman effect refers to the
- a) Splitting of the spectral lines in a magnetic field
- b) Splitting up of the spectral lines in an electrostatic field
- c) Emission of electrons from metals when light falls on it
- d) Random scattering of α-particles by gold foil
Answer: Splitting of the spectral lines in a magnetic field
Question: Number of spectral lines in Balmer series when an electron return from 7th orbit to 1st orbit of hydrogen atom are
- a) 5
- b) 6
- c) 21
- d) 15
Answer: 5
Question: If kinetic energy of a proton is increased nine times, the wavelength of the de-Broglie wave associated with it would become
- a) 3 times
- b) 9 times
- c) 1/3 times
- d) 1/9 times
Answer: 1/3 times
Question: The number of waves in the third orbit of H atom is
- a) 1
- b) 2
- c) 4
- d) 3
Answer: 3
Question: The de-Broglie wavelength of an electron travelling with 10% of velocity of light is equal to
- a) 242.4 pm
- b) 24.2 pm
- c) 2.42 pm
- d) 2.424 pm
Answer: 24.2 pm
Question: The wavelength associated with a ball of 200 g and moving with a speed of 5 m/hour is of the order of
- a) 10–10 m
- b) 10–20 m
- c) 10–30 m
- d) 10–40 m
Answer: 10–30 m
Question: The momentum of a particle which has a de-Broglie wavelength of 0.1 nm is
- a) 3.2 × 10–24 kg ms–1
- b) 4.3 × 10–22 kg ms–1
- c) 5.3 × 10–22 kg ms–1
- d) 6.62 × 10–24 kg ms–1
Answer: 6.62 × 10–24 kg ms–1
Question: The uncertainty in velocity of an electron present in the nucleus of diameter 10–15m hypothetically should be approximately
- a) 10–11 m/s
- b) 108 m/s
- c) 1011 m/s
- d) 10 Å/s
Answer: 1011 m/s
Question: The set of quantum numbers not applicable to an electron
- a)
- b)
- c)
- d)
Answer:
Question: The principal and azimuthal quantum number of electrons in 4f orbitals are
- a) 4, 2
- b) 4, 4
- c) 4, 3
- d) 3, 4
Answer: 4, 3
Question: How many 3d electrons can have spin quantum number 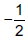 ?
?
- a) 5
- b) 7
- c) 8
- d) 10
Answer: 5
Question: The correct order of increasing energy of atomic orbital is
- a) 5p < 4f < 6s < 5d
- b) 5p < 6s < 4f < 5d
- c) 4f < 5p < 5d < 6s
- d) 5p < 5d < 4f < 6s
Answer: 5p < 6s < 4f < 5d
Question: Which shell would be the first to have ‘g’ sub-shell?
- a) L
- b) M
- c) N
- d) O
Answer: O
Question: For which one of the following set of quantum numbers an electron will have the highest energy?
- a)
- b)
- c)
- d)
Answer:
Question: The energies of orbitals of H-atom are in the order
- a) 3s < 3p < 4s < 3d < 4p
- b) 3s < 3p < 3d < 4s < 4p
- c) 3s = 3p = 3d < 4s = 4p
- d) 3s = 3p = 3d < 4s < 4p
Answer: 3s = 3p = 3d < 4s = 4p
Question: Which of the following set of quantum number is possible?
- a) n = 4, l = 2, m = –2, s = –2
- b) n = 4, l = 4, m = 0, s =1/2
- c) n = 4, l = 3, m = –3, s =1/2
- d) n = 4, l = 0, m = 0, s = 0
Answer: n = 4, l = 3, m = –3, s =1/2
Question: The maximum number of electrons in an atom which can have n = 4 is
- a) 4
- b) 8
- c) 16
- d) 32
Answer: 32
Question: In the presence of magnetic field, the possible number of orientations for an orbital of azimuthal quantum number 3, is
- a) Three
- b) One
- c) Five
- d) Seven
Answer: Seven
Question: Assuming the velocity to be same, the wavelength of the waves associated with which of the following particles would be maximum?
- a) An electron
- b) A proton
- c) An α-particle
- d) A deutron
Answer: An electron
Question: For a ‘p’ electron, the orbital angular momentum is
- a)
- b)
- c) h
- d) 2h
Answer:
Question: Which of the following electronic level would allow the hydrogen to absorb a photon but not emit a photon?
- a) 3s
- b) 2p
- c) 2s
- d) 1s
Answer: 1s
Question: Which of the following transition will emit maximum energy in hydrogen atom?
- a) 4f → 2s
- b) 4d → 2p
- c) 4p →2s
- d) All have same energy
Answer: All have same energy
Question: In an atom, which has 2K, 8L, 18M and 2N electrons in the ground state. The total number of electrons having magnetic quantum number, m = 0 is
- a) 6
- b) 10
- c) 7
- d) 14
Answer: 14
Question: The probability density curve for 2s electron appears like
- a)
- b)
- c)
- d)
Answer:
Question: A p-orbital can accommodate upto
- a) Four electrons
- b) Six electrons
- c) Two electrons
- d) Eight electrons
Answer: Two electrons
Question: If the uncertainty in the position of electron is zero, the uncertainty in its momentum would be
- a) Zero
- b)
- c)
- d) Infinite
Answer: Infinite
Question: The number of radial nodes in 4s and 3p orbitals are respectively
- a) 2, 0
- b) 3, 1
- c) 2, 2
- d) 3, 2
Answer: 3, 1
Question: Which of the following orbital is with the four lobes present on the axis?
- a) dz2
- b) dxy
- c) dyz
- d) dx2-y2
Answer: dx2-y2
Question: Which of the following statement concerning the four quantum number is incorrect?
- a) n gives the size of an orbital
- b) l gives the shape of an orbital
- c) m gives the energy of the electron in orbital
- d) s gives the direction of spin of electron in the orbital
Answer: m gives the energy of the electron in orbital
Question: Which of the following has maximum number of unpaired electrons?
- a) Mg2+
- b) Ti3+
- c) Fe2+
- d) Mn2+
Answer: Mn2+
Question: Two electrons in K shell will not have
- a) Same principal quantum number
- b) Same azimuthal quantum number
- c) Same magnetic quantum number
- d) Same spin quantum number
Answer: Same spin quantum number
Question: The orbital diagram in which both Pauli’s exclusion principle and Hund’s rule are violated is
- a)
- b)
- c)
- d)
Answer:
Question: Which of the following electronic configuration is not possible?
- a) 2p3
- b) 2d5
- c) 4s1
- d) 5f8
Answer: 2d5
Question: Which of the following electronic configuration is incorrect?
- a) 1s2, 2s2, 2px2, 2py2, 2pz2, 3s2, 3px1
- b) 1s2, 2s1, 2px1, 2py1, 2pz1
- c) 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s2
- d) 1s2, 2s2, 2p6, 3s2, 3px1, 3py1, 3pz1
Answer: 1s2, 2s1, 2px1, 2py1, 2pz1
Question: What will be the longest wavelength line in Balmer series of spectrum of H-atom?
- a) 546 nm
- b) 656 nm
- c) 566 nm
- d) 556 nm
Answer: 656 nm
Question: The uncertainty in momentum of an electron is 1 × 10–5 kg-m/s. The uncertainty in its position will be (h = 6.62 × 10–34 kg-m2/s)
- a) 5.27 × 10–30 m
- b) 1.05 × 10–26 m
- c) 1.05 × 10–28 m
- d) 5.25 × 10–28 m
Answer: 5.27 × 10–30 m
Question: In hydrogen atom, energy of first excited state is –3.4 eV. Then find out KE of same orbit of hydrogen atom
- a) +3.4 eV
- b) +6.8 eV
- c) –13.6 eV
- d) +13.6 eV
Answer: +3.4 eV
Question: Maximum number of electrons in a subshell with l = 3 and n = 4 is
- a) 10
- b) 12
- c) 14
- d) 16
Answer: 14
Question: The total number of subshells in fourth energy level of an atom is
- a) 4
- b) 8
- c) 16
- d) 32
Answer: 4
Question: For which of the following sets of four quantum numbers, an electron will have the highest energy?
- a)
n l m s
3 2 1 +1/ 2
- b)
n l m s
4 2 –1 +1/2
- c)
n l m s
4 1 0 – 1/2
- d)
n l m s
5 0 0 –1/2
Answer:
n l m s
4 2 –1 +1/2
Question: A transition element X has a configuration (Ar)3d 4 in its +3 oxidation state. Its atomic number is
- a) 22
- b) 19
- c) 25
- d) 26
Answer: 25
Question: Among the following which one is not paramagnetic? [Atomic numbers; Be = 4, Ne = 10, As = 33, Cl = 17]
- a) Ne2+
- b) Be+
- c) Cl–
- d) As+
Answer: Cl–
Question: Isoelectronic species are
- a) CO, CN–, NO+, C22–
- b) CO–, CN, NO, C2–
- c) CO+, CN+, NO–, C2
- d) CO, CN, NO, C2
Answer: CO, CN–, NO+, C22–
Question: Consider the following sets of quantum number
Which of the following sets of quantum number is not possible?
- a) (i), (ii), (iii) and (iv)
- b) (ii), (iv) and(v)
- c) (i) and (iii)
- d) (ii), (iii) and (iv)
Answer: (ii), (iv) and(v)
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set A |
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set B |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set A |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set B |
| NEET UG Chemistry Biomolecule MCQs |
| NEET UG Chemistry Chemical Bonding MCQs |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set A |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set B |
| NEET Chemistry Chemical Kinetics MCQs Set A |
| NEET Chemistry Chemical Kinetics MCQs Set B |
| NEET UG Chemistry Chemical Kinetics MCQs |
| NEET UG Chemistry Chemical Thermodynamics MCQs |
| NEET Chemistry Chemistry In Everyday Life MCQs Set A |
| NEET Chemistry Chemistry In Everyday Life MCQs Set B |
| NEET UG Chemistry in Everyday Life MCQs |
| NEET Chemistry States Of Matter MCQs Set A |
| NEET Chemistry States Of Matter MCQs Set B |
| NEET UG Chemistry Classification of Elements MCQs |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set A |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set B |
| NEET UG Chemistry D and F Block Elements MCQs |
| NEET Chemistry Electrochemistry MCQs Set A |
| NEET Chemistry Electrochemistry MCQs Set B |
| NEET Chemistry Electrochemistry MCQs Set C |
| NEET Chemistry Environmental Chemistry MCQs Set A |
| NEET Chemistry Environmental Chemistry MCQs Set B |
| NEET UG Chemistry Environmental Chemistry MCQs |
| NEET Chemistry Equilibrium MCQs Set A |
| NEET Chemistry Equilibrium MCQs Set B |
| NEET Chemistry Equilibrium MCQs Set C |
| NEET UG Chemistry Equilibrium MCQs |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set A |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set B |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set A |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set A |
| NEET Chemistry Hydrocarbons MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set C |
| NEET UG Chemistry Hydrocarbons MCQs |
| NEET Chemistry Hydrogen MCQs Set A |
| NEET Chemistry Hydrogen MCQs Set B |
| NEET UG Chemistry Hydrogen MCQs |
| NEET UG Chemistry Isolation of Metals MCQs |
| NEET UG Chemistry Organic Chemistry MCQs |
| NEET UG Chemistry Organic Compounds Containing Halogens MCQs |
| NEET UG Chemistry Organic Compound Containing Nitrogen MCQs |
| NEET UG Chemistry Organic Compounds MCQs |
| NEET UG Chemistry Organic Compounds Containing Oxygen MCQs |
| NEET UG Chemistry P Block Elements MCQs |
| NEET UG Chemistry Practicals MCQs |
| NEET UG Chemistry Redox Reactions and Electrochemistry MCQs |
| NEET UG Chemistry S Block Elements MCQs |
| NEET Chemistry Solutions MCQs Set A |
| NEET Chemistry Solutions MCQs Set B |
| NEET UG Chemistry Solutions MCQs |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set A |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set B |
| NEET UG Chemistry Some Basic Concepts MCQs |
| NEET UG Chemistry States of Matter MCQs |
| NEET Chemistry Structure Of Atom MCQs Set A |
| NEET Chemistry Structure Of Atom MCQs Set B |
| NEET UG Chemistry Structure of Atom MCQs |
| NEET Chemistry Surface Chemistry MCQs Set A |
| NEET UG Chemistry Surface Chemistry MCQs |
| NEET Chemistry The D and F Block Elements MCQs Set A |
| NEET Chemistry The D and F Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set A |
| NEET Chemistry The P Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set C |
MCQs for Structure Of Atom Chemistry Full Syllabus
Expert teachers of studiestoday have referred to NCERT book for Full Syllabus Chemistry to develop the Chemistry Full Syllabus MCQs. If you download MCQs with answers for the above chapter you will get higher and better marks in Full Syllabus test and exams in the current year as you will be able to have stronger understanding of all concepts. Daily Multiple Choice Questions practice of Chemistry will help students to have stronger understanding of all concepts and also make them expert on all critical topics. After solving the questions given in the MCQs which have been developed as per latest books also refer to the NCERT solutions for Full Syllabus Chemistry. We have also provided lot of MCQ questions for Full Syllabus Chemistry so that you can solve questions relating to all topics given in each chapter. After solving these you should also refer to Full Syllabus Chemistry MCQ Test for the same chapter.
You can download the NEET MCQs for Full Syllabus Chemistry Structure Of Atom for latest session from StudiesToday.com
Yes, the MCQs issued by NEET for Full Syllabus Chemistry Structure Of Atom have been made available here for latest academic session
You can find NEET Full Syllabus Chemistry Structure Of Atom MCQs on educational websites like studiestoday.com, online tutoring platforms, and in sample question papers provided on this website.
To prepare for Structure Of Atom MCQs, refer to the concepts links provided by our teachers and download sample papers for free.
Yes, there are many online resources that we have provided on studiestoday.com available such as practice worksheets, question papers, and online tests for learning MCQs for Full Syllabus Chemistry Structure Of Atom

