M.C.Q.
1. Which of the following is a techique of purification of orgainc compound ?
(a) Oxidation (b) Reduction (c) Distillation (d) Hydrolysis
2. Which of the following is/are method (s) of checking the purity of organic compound ?
(a) Chromatography (b) Spectroscopy (c) Both (a) and (b) (d) Neither (a) nor (b)
3. The formula representing a simple ratio of atoms of different elements present in a molecule of the substance is called
(a) Empirical formula (b) Molecular formula (c) Structural formula (d) Condensed formula
4. What is called the purification technique based on difference in the solubilities of the compound and the impurities in a given solvent ?
(a) Sublimation (b) Crystallization (c) Distillation (d) Fractional distillation
5. A compound contains C = 90% and H = 10 %. Find empirical formula of the compound.
(a) C3H10 (b) CH2 (c) C3H2 (d) C3H4
6. Empirical formula of a compound is CH2O and its vapour density is 30. What is the molecular formula of compound ?
(a) C3H6O3 (b) C2H4O2 (c) C2H4O (d) CH2O
7. An organic compound contains C = 36%, H = 6%. What is the empirical formula of compound ?
(a) C3H6 (b) CH3 (c) CH2O (d) C2H2O2
8. What should be the property of solvent used in crystallisation ?
(a) Impure compound should be sparingly soluble in it at room temperature.
(b) Impure compound should be appreciably soluble in it at higher temperture.
(c) Both (a) and (b) (d) Neither (a) nor (b)
9. Which method of purification of organic compound is useful to separate volatile liquids from non volatile impurities ?
(a) sublimation (b) Distillation
(c) crystallisation (d) Fractional crystallisation
10. Which method of purification of organic compound is useful to separate liquids having sufficient difference in their boiling point ?
(a) Sublimation (b) Distillation (c) Filtration (d) Crystallisation
11. An organic compound on analysis gave C = 48gm, H = 8gm and N = 56gm. Volume of 1.0 gm of the compound was found to be 200 ml at STP. What is the molecular formula of the compound ?
(a) C4H8N4 (b) C2H4N2 (c) C12H24N12 (d) C16H32N16
12. Which of the following method can be empolyed to separate a mixture of Chloroform (b.p334k) and aniline (b.p 457k) ?
(a) Distillation (b) Crystallisation (c) Sublimation (d) All of these
13. Insulin contains 3.4% Sulphur. The minimum molecular weight of insulin is
(a) 350 (b) 470 (c) 560 (d) 940
14. Which element is estimated by Carius method ?
(a) Nitrogen (b) Carbon (c) Hydrogen (d) Halogen
15. Fractional distillation is used when,
(a) Difference in melting points of two solids is less.
(b) Difference in boiling points of two liquids is large.
(c) Difference in boiling points of two liquids is small.
(d) Difference in melting points of two liquids is large.
16. Distillation under reduced pressure is used when_____
(a) liquid has very high boiling point and decompose at or below its boiling point.
(b) liquid has very low boiling point.
(c) liquid is insoluble in water.
(d) liquid is insoluble in organic solvent.
17. In Carius method, 0.099 g compound gave 0.287 gm AgCl. What is the % of Cl ?
(a) 35.4 (b) 71.7 (c) 46.2 (d) 80.5
18. In which compound, sulphur of organic compound is converted when organic compound is reacted with con. HNO3 for estimation of Sulphur ?
(a) H2SO4 (b) SO2 (c) H2S (d) SO3
19. Which of the following techniques is used to separate glycerol from spent-lye in soap industry ?
(a) Simple distillation (b) Fractional distillation
(c) Distillation under reduced pressure (d) None of these
20. Which method is used to separate aniline water mixture ?
(a) steam distillation (b) distillation under reduced pressure
(c) sublimation (d) None of these
21. Chromatography is used
(a) to separate mixtures (b) to purify compounds
(c) to test the purity of compounds (d) All of these
22. In chromatography Rf means
(a) Retardation factor (b) Reduced formula
(c) Reduction factor (d) Retardation frequency
23. Statements regarding Chromatography are given below. Identify True or False statements and give your answer.
(1) Silica - gel is used as adsorbent in TLC. (2) Rf = Retardation factor.
(3) Adsorbent is a mobile phase. (4) Chromaplate is not a part of TLC.
(a) TTFF (b) TTTT (c) TFFT (d) TFTF
24. In Chromatography, ninhydrin solution is used to detect
(a) amino acids (b) ethers (c) hydrocarbons (d) glucose
25. In a method of detecting C and H, organic compound is heated with ____
a) CuO (b) Ca(OH)2 (c) CuSO4 (d) All of these
26. In a detection of C and H, the water produced is tested by
(a) CuO (b) Ca(OH)2 (c) CuSO4 (d) All of these
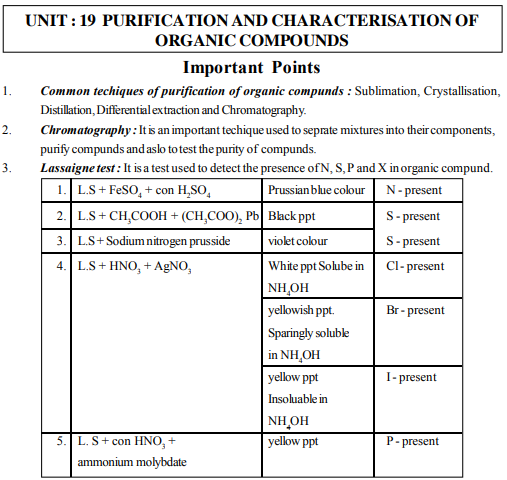
27. Lassaigne test is used for detection of
(a) N (b) S (c) P (d) All of these
28. Lassaigne test is used for detection of
(a) S (b) P (c) X (d) All of these
29. The basic principle of Lassaigne test is
(a) to convert elements present in compound from covalent form to ionic form.
(b) to convert elements present in compound from ionic form to covalent form.
(c) to convert elements present in compound into their complexes
(d) None of the above.
30. The prussian blue colour obtained in Lassaigne test is due to
(a) Na4 [Fe(CN)6] (b) Fe4[Fe(CN)6] (c) CuSO4 (d) FeSO4
31. In the Lassaigne test of detection of Sulphur, black precipitates are obtained. These are due to the formation of
(a) Na2S (b) (CH3COO)2Pb (c) PbS (d) CH3COOH
32. What colour is observed when Lassaigne solution of Suplhur containing compound is treated with Sodium Nitroprusside ?
(a) Red (b) Violet (c) Blue (d) Brown
33. In a Lassaigne test of Sluphur containing compound the violet colour is due to the
(a) [Fe(CN)5NO]2- (b) [Fe(CN)5NOS]4- (c) Na4[Fe(CN)6] (d) K4[Fe(CN)6]
34. During the estimation of C and H, the CO2 produced id absorbed in
(a) KOH (b) An.CaCl2 (c) CuO (d) O2
35. During the estimation of C and H, the H2O produced id absorbed in
(a) KOH (b) An.CaCl2 (c) CuO (d) O2
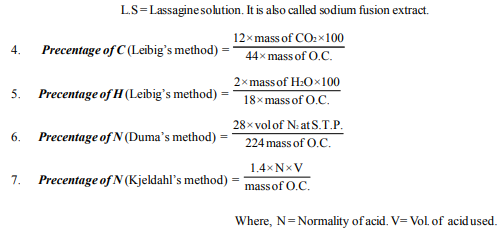
36. In Kjeldahl’s method for the estimation of nitrogen, the formula used is
37. An organic compound on analysis give the following result : C = 54.5%, O = 36.4%, H = 9.1%. The empirical formula of the compound is
(a) CH3O (b) C2H4O (c) C3H4O (d) C4H8O
38. An organic compound gave the following results on analysis. C = 53.3%, H = 15.6%, N = 31.1%. Find molecular formula of compound. (Molecular Weight = 45)
(a) C2H5N2 (b) C2H7N (c) C2H5N (d) C2H6N
39. An organic compound contains C, H and O in the proportion of 6 : 1 : 8 by weight, respectively. Find its molecular formula (V.D = 30)
(a) CH2O (b) C3HO (c) C2H4O2 (d) CH4O
40. 64 gm of organic compound contains 24 gm C, 8 gm H and the rest oxygen. What is the empirical formula of the compound ?
(a) CH2O (b) CH4O (c) C2H4O (d) C2H8O2
41. 0.2595 gm of organic substance gave 0.35 gm BaSO4. Find % S in the substance.
(a) 18.5 (b) 182 (c) 17.5 (d) 175
42. Find the incorrect formula
43. On complete combustion, 0.246g organic substance gave 0.198 g CO2 and 0.1014 g H2O. Find % of C and H in compound ?
(a) 4.58, 21.95 (b) 21.95, 4.58 (c) 19, 21 (d) 21, 19
44. Which of the following is the method of estimating nitrogen in organic Compound ?
(a) Dumas method (b) Kjeldahlis method (c) Both (A) & (B) (d) Neither(a) nor (b).
45. In the Dumas method nitrogen containing organic compound is first heated with
(a) O2 (b) CuO (c) CO2 (d) CuO and CO2
46. In Dumas method the gaseous mixture produced is passed through KOH(aq ). As a result of this _______ is absorbed in KOH(aq).
(a) CO2 (b) O2 (c) NOx (d) All of these
47. A device called nitrometer is used in
(a) Dumas method (b) Kjeldahl’s method (c) Carius method (d) Leibig’s method
48. In Dumas method the formula of % N is
49. In Dumas method for estimation of nitrogen, 0.3g of an organic compound gave 50 ml of nitrogen collected at 300K temperature and 715 mm pres sure. Calculate the % of N in the compound. ( Aqueous tension at 300K = 15mm)
(a) 20.8 % (b) 17.46 % (c) 34.5% (d) 25 %
50. In Kjeldahl’s method, when nitrogenous organic compound is heated with con. sulphuric acid, the nitrogen is converted into
(a) (NH4)2 SO4 (b) NH4OH (c) NH3 (d) None
53. During estimation of nitrogen present in organic compound by Kjeldahl’s method, the ammonia cvolved from 0.5 gm of the compound neutralized 10 ml of 1 M H2SO4 Calculate the %N in the compound.
(a) 65 (b) 28 (c) 56 (d) 30
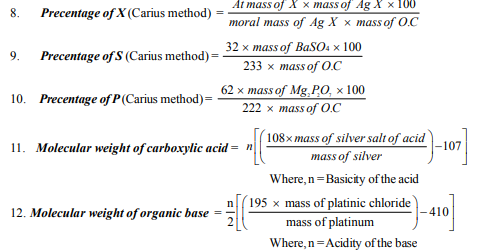
54. In the estimation of halogen by Carius method, the halogen of the organic compound is converted into
(a) Silver nitrate (b) Silver halide (c) Silver Sulphate (d) Silver oxide
55. In Carius method of estimation of halogen, 0.15 gm of an organic compound gave 0.12gm of AgBr. Calculate % of bromine in the compound.
(a) 34.04% (b) 17.02% (c) 53.19% (d) 18.8%
56. In estimation of Sulpur in organic compound, the amount of sulphur is determined in terms of
(a) H2SO4 (b) BaSO4 (c) SO2 (d) SO3
57. In estimation sulphur, 0.157gm of an organic compound gave 0.4813 gm of barium Sulphate.
Calculate % S in compound.
(a) 24.10 % (b) 14.20% (c) 42.10% (d) 38%
58. In estimation of phosphorous present in organic compound, the amount of phosphorous is determined in terms of
(a) Phosphoric acid (b) Ammonium molybdate
(c) Ammonium phospho molybdate (d) Magnesium pyrophosphate
59. Suggest a method to purify camphor containing impurity of comman salt.
(a) Crystallisation (b) Fractional crytallisation
(c) Sublimation (d) Distillation
60. Suggest a method to separate a mixture of ortho and para nitro aniline.
(a) Distillation (b) Chromotography
(c) Differential extraction (d) All of these
61. Suggest a method to separate a mixture of methanol and acetone.
(a) Simple distillation (b) Fractional distillation
(c) Fractional Crystallisation (d) Differential extraction
62. Suggest a method to purify napthalene.
(a) Sublimation (b) Crystallisation
(c) Differential extraction (d) Distillation
63. Suggest a method to separate a mixture containing acetone and ethanol
(a) Sublimation (b) Crystallisation
(c) Differential extraction (d) Distillation
64. Which of the following compound will not give positive Lassaigne test for nitrogen ?
(a) Hydrazine (b) urea (c) ethylamine (d) nitroethane.
65. Name a suitable technique of separation of a mixture of calcium sulphate and camphor.
(a) Crystallisation (b) Sublimation (c) Distillation (d) None
66. The fragrance of flower is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour. A suitable method for the extraction of these oils from the flower is,
(a) Distillation (c) Crystallisation
(c) Distillation under reduced pressure (d) Steam distillation
67. Kjeldahl’s method can not be used for estimation of nitrogen in____
(a) DNA (b) RNA (c) a and B (d) Neither a and b
68. Name the method used to separate a mixture of iodine and sodium chloride ?
(a) Crystallisation (b) Fractional Crystallisation
(c) Sublimation (d) Steam distillation
69. What conclusion can be drawn if red colour is obtained during Lassaigne test ?
(a) Pressure of S (b) Pressure of N
(c) both a and b (d) Neither a and b
70. What is the formula of iron (III) hexacyanoferrate (II) ?
(a) Fe4[Fe(CN)6]3 (b) Fe3[Fe(CN)6]4 (c) Fe4[Fe(CN)6] (d) Fe3[Fe(CN)6]
71. Two volatile liquids A and B differint their boiling points by 15K. Which process can be used for their sepration ?
(a) Fractional distillation (b) Steam distillation
(c) Distillation under reduced pressure (d) Simple distillation
72. An organic compound from its aqueons solution can be seprated by
(a) Distillation (b) Solvent extraction
(c) Fractional distillation (d) Steam distillation
73. The ammonia evolved from the treatment of 0.3gm of organic compound for estimation of nitrogen was passed in 100ml of 0.1m H2SO4. The excess acid required 20ml 0.5 M NaOH for complete neutralisation The organic compound is___
(a) acetamide (b) benzamide (c) urea (d) thiourea
74. Which of the following compound gives blood red colouration when its Lassaigne extract is treated with alkali and ferric chloride ?
(a) Thiourea (b) Diphenyl Sulphide
(c) Phenyl hydrazine (d) Benzomide
75. Which of the following compounds will give Lassaigne test for nitrogen ?
(a) NH2NH2 (b) NaNO3 (c) NH4Cl (d) NaCN
76. Which of the folowing will give bloodred colour while doing Lassaigne test for nitrogen
(a) urea (b) Benzene sulphonic acid
(c) Hydrazine (d) p-aminobenzene sulphonic acid
77. Name the method used to determine the molecular weight of organic compound.
(a) Silver salt method (b) Chloroplatinate salt method
(c) Mass spectrometery (d) All of these
78. While detecting phosphorous in organic compound yellow colour is obtained due to the formation of
(a) (NH4)2MoO4 (b) (NH4)3PO4 (c) (NH4)3PO4 . 12MoO3 (d) MgNH4PO4
79. 0.306 gm of the silver salt of a monobasic organic acid yeilds 0.216gm of Silver on ignition.
Find molecular weight of the acid.
(a) 46 (b) 23 (c) 122 (d) 20
80. 1.026gm of the silver salt of a tribasic organic acid gave an ignition 0.648gm silver. Find molecular weight of the acid.
(a) 148 (b) 192 (c) 208 (d) 234
81. 0.3557gm of the Platinum salt of monoacidic organic base yeilds 0.117gm of platinum. Find molecular weight of base
(a) 95 (b) 91.45 (c) 98 (d) 410
82. Chromatography is used for the purification of
(a) solids (b) liquids (c) gases (d) All of these
83. Which of the following reagents is used for detection of unsaturation in alkenes.
(a) NaOH + CaO (b) cold dil, alkaline kMnO4
(c) Cl2/hv (d) KOH / EtOH
84. The presence of halogen in an organic compound is detected by
(a) Iodo form test (b) Silver mirror test
(b) Beilstein’s test (d) Millon’s test
87. How will you separate a solution of benzene and chloroform ?
(a) Sublimation (b) Filtration (c) Distillation (d) Crystallisation
88. When 32.25 gm ehtyl chloride dehydrohalogenated it gives 50% alkene. What is the mass of product ?
(a) 7 gm (b) 14 gm (c) 21 gm (d) 28gm
89. How much sulphur is present in organic compound if on analysis 0.53gm of this compound gives 1.158gm of BaSO4 ?
(a) 10% (b) 20% (c) 30% (d) 40%
90. Which method can be used to separate a mixture of KCl and KClO3 ?
(a) Crystallisation (b) Fractional Crystallisation
(c) Sublimation (d) Distillation
91. Middle ton’s test is used to detect
(a) N (b) P (c) X (d) S
92. An organic compound weighing 4.337mg yielded 10.35mg of CO2 and 3.42mg of H2O on oxidation with CuO. Calculate the empirical formula of the compound.
(a) C3H5 (b) C3H5O (c) C4H6O (d) C10H16O3
93. An organic compound contains 69% C and 4.8% H, the remainder being oxygen. Calculate the masses of CO2 and H2O produced when 0.2gm of this substance is completely combusted.
(a) 0.506g & 0.864g (b) 0.0864g & 0.0506g
(c) 0.506g & 0.0684g (d) 0.506g & 0.0864g
94. 0.3780 gm of chlorine containing organic compound gave 0.5740gm of silver chloride in Carius estimation. Calculate the % of Cl.
(a) 37.56% (b) 40.5% (c) 35.7% (d) 31.23%
95. In the estimation of sulphur by Carius method, 0.468gm of organic compound gave 0.668gm BaSO4. Calculate % of S.
(a) 19.6 (b) 21.7 (c) 17.9 (d) 16.9
In each of the following questions, a statement (A) is given followed by Reason (R).
Give your answers as
(A) If both A and R are true, and reason is the true explanation of the assertion.
(B) If both A and R are true, but R is not the true explanation of the A.
(C) If A is true but R isfalse.
(D) If both A and R are false.
96. A: Vapour density and density both are same
R: Molecular weight is twice the density
97. A: Lassaigne’s extract is boiled with dil.HNO3 before testing for halogens by AgNO3.
R: CN- and S2- present in Lassaigne extract interfere with the test of halide ions by silver nitrate.
98. A: Lassaigne’s test is shown by diazonium salts.
R: Diazonium salts are volatile.
99. A: Essential oils are purified by steam distillation.
R: Essential oils are volatile and are insoluble in water.
100. A: Sulphur present in organic compound can be estimated quantitavely by Carius method.
R: Sulphur is separated easily from other atoms in the molecules and gets precipitated as light yellow solid.
ANSWER KEY

