
M.C.Q.
1. Identify the wrong statement in the follwing
(a) CFCs are responsible for ozone layer depletion.
(b) Greenhouse effect is responsible for global warming.
(c) Ozone layer does not permit I.R. radiation from the sun to reach the earth.
(d) Acid rain is mostly because of oxides of ‘N’ and ‘S’.
7. Number of atoms in 560 g of Fe (atomic mass = 56) is
(a) twice that of 70 g N. (b) half that of 20 g H
(c) both (a) and (b) (d) None of these
11. An Oxide of metal contains 60% of the metal. What will be the equivalent weight of the metal ?
(a) 12 (b) 40 (c) 24 (d) 48
12. A container is filled with 2L of water. What will be the volume of water in m3?
(a) 2 ×103 (b) 1×103 (c) 2 ×10-3 (d) 1×10-3
13. The mass of carbon -12 atom considered in the definition of a mole is
(a) 0.012Kg (b) 0.12g (c) 120 mg (d) None of these
14. The drug which is used for treating AIDS victims is
(a) Azidothymidine (b) Cis- platin
(c) Taxol (d) All of these
15. Chose the incorrect statement .
(a) The constituents of a compound cannot be separated into simpler substances by physical methods.
(b) An element is consists of only one type of particles and these particles may be atoms or molecules.
(c) The properties of a compound are same as its constituent elements.
(d) Atoms of different elements are different in nature.
16. Which of the following is a pair of physical and chemical property respectively of a substance ?
(a) acidity & combustibility (b) colour & density
(c) basicity & colour (d) density & acidity.
17. What is the symbol of S.I. unit for the amount of substance ?
(a) NA (b) n (c) mole (d) mol
18. What is the symbol of a multiple ‘109’ ?
(a) G (b) E (c) n (d) Z

22. How many significant digits are there in 0.25 ?
(a) 1 (b) 2 (c) 3 (d) cannot be determined.
23. Which of the following number contains there significant digits ?
(a) 0.200 (b) 0.030 (c) 0.0052 (d) 0.002
24. What is the number of neutrons in Zn2+ ion (Atomic mass namber = 70)
(a) 34 (b) 36 (c) 38 (d) 40
25. The same amount of Zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide.
What will be the ratio of volumes of hydrogen evolved ?
(a) 1 : 1 (b) 1 : 2 (c) 2 : 1 (d) 9 :4
26. 2.76g of silver carbonate on being strongly heated yields a residue weighing
(a) 2.16g (b) 2.48 g (c) 2.32 g (d) 2.64 g
27. Find the total number of electrons in one molecule of carbon dioxide.
(a) 22 (b) 44 (c)66 (d) 88
28. A gaseous mixture contains oxygen and nitrogen in the ratio of 1 : 4 by weight. Therefore, the ratio of their number of molecules is
(a) 1 : 4 (b) 1 : 8 (c) 7 : 32 (d) 3 : 16
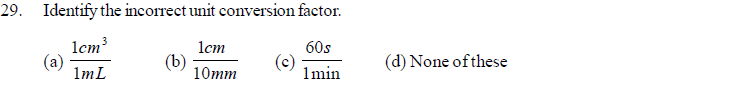

41. Some statements are given below based on the pictures. Identify true and false statements.
(i) ‘P’ and ‘Q’ both indicates precision and accuracy.
(ii) ‘Q’ indicates precision and accuracy white ‘R’ indicates neither precision nor accuracy.
(iii) ‘P’ indicates precision but not accuracy.
(iv) ‘Q’ indicates both precision and accuracy
(a) FTTT (b) TTTT (c) TTFT (d)FTFT
42. The normality of 0.3M phosphorous acid is
(a) 0.1 (b) 0.9 (c) 0.3 (d) 0.6
43. An aqueous solution of 6.3g oxalic acid dihydrate is made upto 250 mL. The volume of 0.1 N NaOH required to completely neutralize 10 mL of this solution is
(a) 40 mL (b) 20 mL (c) 10 mL (d) 4 mL
49. Haemoglobin present in blood contain 3.72% by mass iron. Calculate the number of iron atoms in 2.0g of haemiglobin.
(a) 4.53 X 1026 (b) 4.53 X 1023 (c) 5.95 X 1019 (d) 8 X 1020
50. How many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms
(a) 0.02 (b) 3.125 x 10-2 (c) 1.25 x 10-2 (d) 2.5 x 10-2
51. The unit J Pa -1 is equivalent to
(a) m3 (b) cm3 (c) dm3 (d) none of these
52. The density of Al metal is 2.7 gcm-3. An irregularly shaped piece of aluminium weighing 40.0g is added to a 100mL graduated cylinder containing 50.0mL of water. upto what height the water level will rise in the cylinder ?
(a) 14.8mL (b) 79.6mL (c) 64.8mL (d) 50mL
53. A sample of clay after drying partially was found to contain 50% silica and 7% water.The original sample of clay had 12% water, What is the percentage of silica in the original sample?
(a) 50% (b) 5% (c) 43% (d) 47%
54. In which of the following pairs percent compostion of element is not same ?
(a) benzene and ethyne (b) But - 2 - ene and Cyclobutane
(c) glucose and fructose (d) phenol and ethanol
55. What weight of CuO will be required to provide 200Kg copper
(a) 200Kg (b) 79.5Kg (c) 250Kg (d) 100Kg
56. Choose the proper option after studying following statement (T = True, F = False)
1. The percent composition of vinyl chloride and its polymer PVC are same.
2. The perecent compostion of phosphorous trioxide (P2O3)is half than that of its dimer phosphorous hexoxide (P4O6) for each of the elements present in them.
(a) T, F (b)F,T (c) T, T (d) F, F
57. Impure sample of ZnS contains 42.34% Zn. What is the percentage of pure ZnS in the smaple ?
(a) 67% (b) 63% (c) 58% (d) 37%
58. If the atomic mass of carbon were set at 50 amu, what would be the value of Avog adro’s number ?
(a) 5.01 x 1024 (b) 6.022 x 1023 (c) 1.66 x 1024 (d) none of these
59. For which of the following compounds molecular weigh cannot be determined from atomic weights ?
(a) Fe4[Fe(CN)6] (b) TiO2
(c) TiO1.12 (d) none of these
60. Which one of the following contains greatest number of oxygen atoms ?
(a) 1.0g of O atoms (b) 1.0g of O2
(c) 1.0g of O3 (d) All have same number of atoms
71. Which one of the following statements is incorrect ?
(a) All elements are homogeneous system
(b) Compounds made up of a number of elements are heterogeneous.
(c) A mixture is not always heterogeneous
(d) Smoke is a heterogeneous mixture.
72. A balanced chemical equation is in accordance with
(a) Avogadro’s law.
(b) Law of constant proportions
(c) Law of conservation of mass
(d) Law of gaseous volumes.
(a) i - q, ii - p, iii - r, iv - s (b) i - s, ii - q, iii - p, iv - r
(c) i - q, ii - s, iii - p, iv - r (d) i - r, ii - s, iii - p, iv - q
79. The total number of atoms of all elements present in mole of ammonium dichromate is
(a) 19 (b) 6.023 x 1023 (c) 114.47 x 1023 (d) 84 x 1023
80. 0.32 gm of a metal on treatment with an acid gave 112 mL of hydrogen at STP. Calculate the equivalent weight of the metal
(a) 58 (b) 32 (c) 11.2 (d) 24
81. For a reaction A + 2B → C, the amount of C formed by starting the reaction with 5 moles of A and 8 moles of B is
(a) 5 moles (b) 8 moles (c) 16 moles (d) 4 moles
82. 100 mL of PH3 on heating forms P and H2 . The volume change in the reaction is
(a) an increase of 50 mL (b) an increase of 100 mL
(c) an increase of 150 ml (d) a decrease of 50 mL
83. An organic compound made of C, H, and N contains 20% nitrogen. Its molecular weight is
(a) 70 (b) 140 (c) 100 (d) 65
88. Which of the following is not a homogeneous mixture ?
(a) smoke (b) air (c) Brass (d) Aqueous solution of sugar
89. Which of the following has the largest number of atoms ?
(a) 0.5g atom of Cu (b) 0.635g of Cu
(c) 0.25 moles of Cu atom (d) 1 g of Cu
90. 27 g of A1 (at mass = 27) will react with oxygen equal to
(a) 24 g (b) 8 g (c) 40 g (d) 10 g
91. Two containers P and Q of equal volumes contain 6 g of O2 and SO2 respectively at 300K and 1 atmosphere. Then
(a) No. of molecules in P is less than that in Q
(b) No. of molecules in Q is less than that in P
(c) No. of molecules in P and Q are same.
(d) cannot be determined
92. Which of the following pairs of substances illustrates the law of multiple proportions ?
(a) CO and CO2 (b) NaCl and NaBr
(c) H2O and D2O (d) MgO and Mg(OH)2
In each of the follwoing questions, two statements are given, one is Assertion (A) and the other is Reason (R). Examine the statements carefully and mark the correct answer according to the instructions given below :
(a) If both A and R are correct and R is the correct explanation of A.
(b) If both A and R are correct and R is not the correct explanation of A.
(c) If A is correct R is wrong.
(d) If both A and R are false.
93. A : Normality of 0.1M H2SO4 is 0.2N.
R : H2SO4 is a dibasic acid.
94. A : 1 Gram molecule of sulphar also represents 1 gram atom of sulphur.
R : Atomicity of sulphur is one.
95. A : In the equation NH3 + HCl → NH4 Cl , Gay-Lussac’s law is not applicable to NH4Cl.
R : NH4Cl is not a gas,.
96. A : Atomic mass of sodium is 23 u.
R : An atom of sodium is 23 times heavier than an atom of 12C.
97. A : Pure water, irrespective of its source always contain hydrogen and oxygen in the ratio 1 : 8 by mass.
R : Total mass of reactants and products remains constant during physical or chemical change.
98. A : Mass numbers of most of the elements are fractional.
R: Mass numbers are obtained by comparing with mass number of 12C.
99. A : The mass of the products formed in a reaction depends upon the limting reactant.
R : Limting reactant reacts completely in the reaction.
100. A : Cinnabar is a chemical compound whereas brass is mixture.
R : Cinnabar always contain 6.25 times mercury than sulplur by weight. Brass can have any proportion of Cu and Zn.
101. A : 1 L of O2 and 1 L of O3 contains the same number of moles under identical conditions.
R : Under identical conditions, 1 L of O2 and 1 L of O3 contain the same number of oxygen atoms.
102. A : The standard unit for expressing atomic mass is amu.
R : Now a days amu is represented by ‘u’.