NEET UG Chemistry Hydrocarbons MCQs. NEET UG students can acces the biggest database of MCQs on Studiestoday.com. This collection of MCQs have been prepared by the best NEET teachers in the country. NEET students should download and practice these questions to get better marks in examinations.
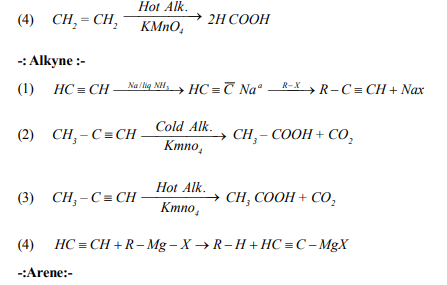
M.C.Q.
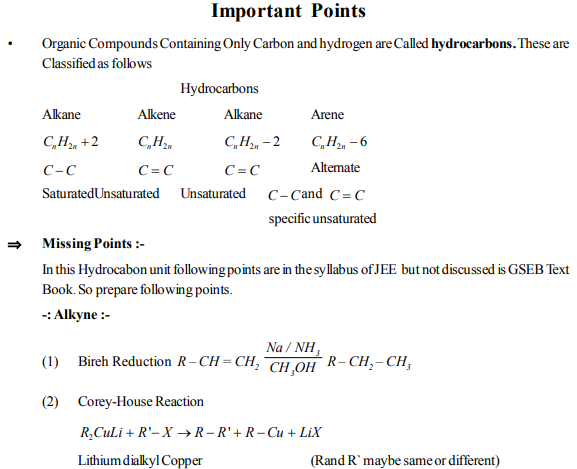
(1) Chloroethane reacts with Na in Presence of dry ether. The Product is
(a) Ethane (b) Propane (c) Butane (d) Ethene
(2) Which represents an alkyne ?
(a) C5H10 (b) C5H12 (c) C3H8 (d) C4H6
(3) If Sodium Propionate is heated with sodium propionate then what will be the product ?
(a) Ethane (b) Propane (c) Propionic acid (d) Propene
(4) Electrolysis of aqueous solution of sodalime then what will be the product ?
(a) Methane (b) Ethane (c) Propane (d) Butane
(5) The preparation of ethane by electrolysis of aqueous solution of sodium acetate is known as ?
(a) Grignard reaction (b) Wurtz reaction (c) Kolbe`s synthesis (d) Frankland reaction
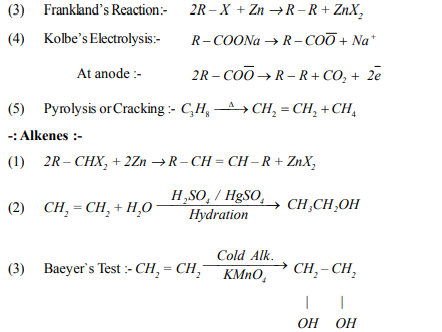
(6) The highest boiling point is expected for
(a) n-butane (b) n- pentane (c) iso-Pentane (d) neo - pentane
(7) Which one of the following has the lowest boiling point
(a) n-butane (b) 2-methyl butane (c) 2-methyl propane (d) n- pentane
(8) Which of the following reactions will not give propane
(9) Halogenation of alkane is an example of ?
(a) Electrophilic Substitution (b) Nucleophilic substitution
(c) Free redical substitution (d) Addition reaction
(10) When ethyl iodide and propyliodide react with Sodium in presence of ether they form ?
(a) Only One alkane (b) Mix ture of two alkane
(c) Mix ture of three alkane (d) Mix ture of four alkane
(11) CH3CH2OH + CH3MgBr→Product .
Product in above reaction is
(a) Methane (b) Ethane (c) Propane (d) Butane
(12) LPG is a mixture of ?
(a) CH4 + C2H6 (b) C3H8 + C4H10 (c)C2H4 + C2H2 (d) C6H6 + C6H12
(13) Aqueous solution of the which of following compound on electrolysis gives ethane ?
(a) Sodium formate (b) Sodium acetate (c) Ethanoicacid (d) Ethylacetate
(14) As the number of branches in a chain increases the boiling point of alkane____
(a) Increases (b) Decreases (c) Remains same (d) May increases or decreases
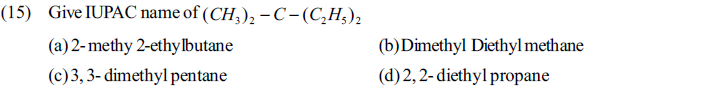
(16) Which conformational isomer of ethane is more stable ?
(a) Skew (b) Staggered(Anti) (c) Partially eclipseds (d) Fully eclipsed
(17) Which Conformational isomer of Cyclohexane is more stable ?
(a) Chair (b) Twist boat (c) boat (d) half chair
(18) The number of possible structural isomer of heptane is.
(a) 8 (b) 9 (c) 10 (d)12
(19) Formation of alkane by the action of Zn on alkyl halide is called ?
(a) Frankland`s reaction (b) Wurtz reaction
(c) Cannizzaro reactions (d) Kolbe`s reactions
(28) Methyl chloride react with Lithium diethyl Copper to give___
(a) Ethane (b) Propane (c) Butane (d) Propene
(29) Which of the following alkyl bromides may be used for the synthsis of 2,3-dimethyl butane by Wurtz raection ?
(a) n- propyl bromide (b) iso-propyl bromide (c) n-butyl bromide (d) iso-butyl bromide
(38) when 3-phenyl propene reacts with HBr in the presence of peroxide, the major product form is
(a) 2-bromo-1-phenyl propane (b) 1,2-dibromo-3-phenyl propane
(c) 3-(0-bromo phenyl )propane (d) 1-bromo-3-phenyl propane
(39) Baeyer`s Test is used in the laboratory for
(a) Detection of alcohol (b) Detection of double bonds
(c) Detection of Glucose (d) Detection of amines
(40) Aqueous H2SO4 reacts with 2-methyl-but-1-ene to give predominantly
(a) 2-methyl-butane -1-ol (b) 2-methyl-butane -2–ol
(c) Isobutyl Hydrogen sylphate (d) Sec. butyl hydrogen sulphate
(59) The dehydrohalogenation of neopentylbromide mainy gives
(a) 2-methyl-but-1-ene (b) 2-methyl-but-2-ene
(c) But-2-ene (d) 2,2- dimethyl-but-1-ene
(60) 1,3-butadiene (Buta-1,3-diene) reacts with ethylene to form
(a) Benzene (b) Cyclohexane (c) Cyclohexene (d)2,3-dimethyl butane
(61) Which of the following represents the given mode of hybridization SP2 - SP2 - SP - SP from left to right
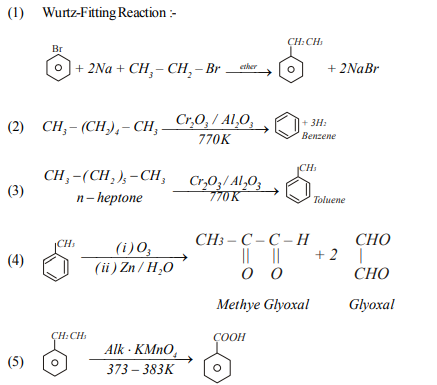
(91) Point out the wrong statement in relation to the structure of Benzene.
(a) It forms only one monosubstitated derivative
(b) The C-C bond lenght is benzene is uniformly1.397 A0
(c) It is a resonance hybrid of a number of canonical forms
(d) It has three delocalised π- molcular orbitals
(92) Which of the following when treated with super heated steam under pressure gives benzene?
(a) Benzene Sulphonic acid (b) Benzyl Chloride
(c) Bromo Benzene (d) Nitro benzene
(95) Which is not aromatic hydrocarbon?
(a) Benzene (b) Toluene (c) phenol (d) Napthalene
(96) Which of the following is not aromatic ?
(a) Benzene (b) Cyclopropenyl cation
(c) Trophylium cation (d) Cyclopentadienyl cation
Assertion and Reason type Questions
Direction:- In each of the following Questions Read the Assertion(A) and Reason (R)
carefully. Choose Correct option as under and darken trhe bubbles in OMR.
(A) If both A and R are true and R is the Correct explanation of A
(B) If both A and R are true but R is not the Correct explanation of A
(C) If A is true but R is false
(D) If both A and R are false
(E) If A is false but R is ture
(131) A : Pyrrole is an aromatic heterocyclic compound
R : It has a cyclic, delocalised 6pelectrons
(132) A : CH4 does not react with Cl2 in dark
R : Chlorination of CH4 takes place in Sunlight
(133) A : Neopentane forms only one monosubstitated Compound
R : Neopentane has high bond energy
(134) A : Addition of HBr to propene in presence of peroxide gives 1- bromo propane.
R : The reaction occurs by Free radical mechanism
(135) A : Styrene on reaction with HBr gives 2-bromo-2-phenylethane
R :Benzyl radical is more stable than alkyl radicals
(136) A : Butane-1-ol on dehydration Conc.H2SO4 gives mainly But-1-en
R :Dehydration occurs through Carbocation intermediate
(137) A : Melting point of neopentane is higher than that of iso-pentane
R : Neopentane Contains a quaternary carbon
(138) A : Acetylene react with NaNH2 to give Sodium acetylide and ammonia
R : SP-hybridized carbon of acetylene are considerably electronegative
(139) A : Addition of H2O to acetylene occurs in presence of dil.
H2SO4 and HgSO4 to give acetaldehyde
R : It is an example of electrophilic addition reaction
(140) A : Addition of HI to vinyl chloride produces 1-chloro1-iodoethane
R : HI adds to vinyl chloride againt markownikov`s Rule
Matrix Match Type Questions
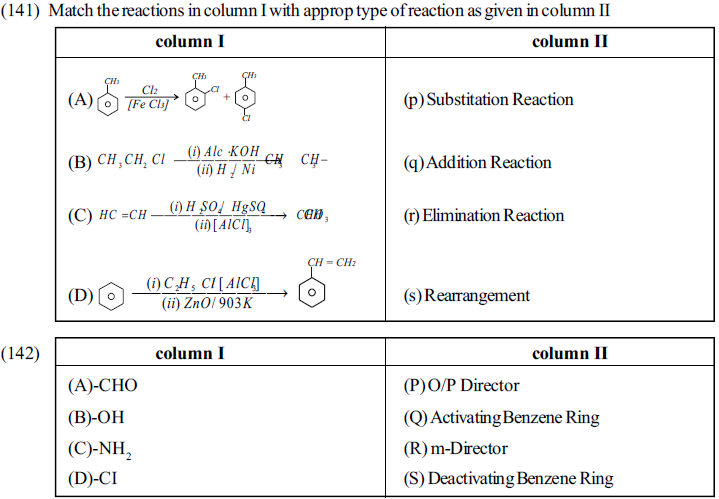
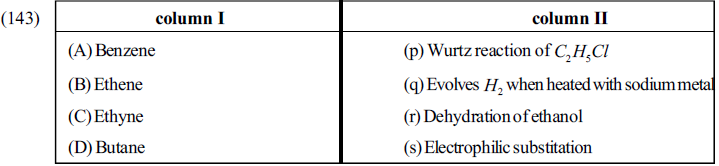
Comprehension Type Questions
Direction :- Question numbers 144 and 145 are based on the following paragraph. Each question has 4 options (A),(B),(C) , (D) out of which ONLY ONE is correct. choose the correct option.
Paragraph :- Cyclohexene on ozonolysis following by reaction with Zinc dust and water gives aldehyde(P). Compound (p) on further treatment with aqueous KOH yields compound(Q).

