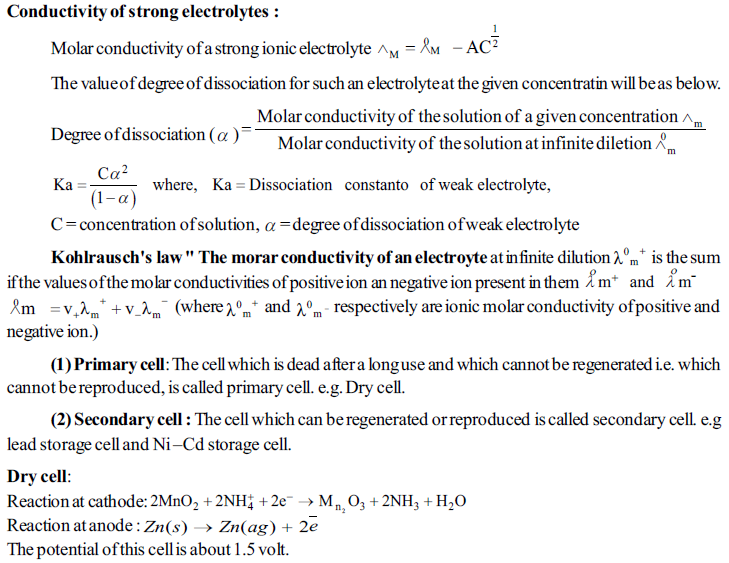
IMPORTANT POINTS
Faraday's laws of Electrolysis:
(i) First law: The amount of products produced at the electrodes by electroysis are directly proportional to the quntity of the electricity passed through the electroytic cell. if w is the mass of the product produced and Q is value of the quntity of electricity passed, than W α Q
(ii) Second law : If the different electroytic cells, containing different electrolysis are joined in series and same quntity of electricity os passed through them, than the amount of products obtained at the electrodes are directly proportional to thier equivalent weight.
W α Eq, where W = Mass of product obtained and Eq = Equivalent weifht of product. the modern presentation of Faraday's law was made as follows:
"The products, obtained at the electrodes by oxidation and reduction half– reactions have the relation with the moles of the products and stoichiometry of the reaction and the quntity of electricity." " The products, obtained at the electrodes by oxidation and reduction half– reactions have the relation with the moles of the products and stoichiometry of the reaction and the qunantity of electricity."
The quantity of electricity passed by 1 more electrons is called one Faraday.
M.C.Q.
1. Reduction reaction means ________
(a) a process of adding oxygen (b) a process of removing hydrogen
(c) a process of adding electron (d) a process of removing electrons
2. Which substance is oxidizing agent ?
(a) a substance donates hydrogen or accepts oxygen
(b) a substance donates oxygen or accepts hydrogen
(c) a substance experience oxidation
(d) a substance donates electron
3. Which substance is called reducing agent ?
(a) a substance donates hydrogen or accepts oxygen
(b) a substance accepts hydrogen or donates oxygen
(c) a substance expereince reduction (d) a substance gains electron
4. Oxidation reaction means _____________
(a) a process of removing electron (b) a process of adding hydrogen
(c) a process of removel of oxygen (d) a process of adding electrons
5. Which of the following is the characterictic of reducing agent ?
(a) it experience oxidation. (b) it experience reduction
(c) it gains electrons (d) it gives oxygen
6. Which of the following is the characteristic of oxidizing agent ?
(a) it experience oxidation. (b) it experience reduction
(c) it gains oxygen (d) it donates electrons
7. Which of the following statement is true ?
(a) there is always reduction occur of oxidizing agent
(b) there is always oxidation occur of reducing agent
(c) oxidation and reduction are supplimentary processes
(d) Given three statements are wrong.
8. Which of the following statement is wrong ?
(a) there is always reduction occur of oxidizing agent
(b) there is always oxidation occur of reducing agent
(c) oxidation and reduction are supplimentary processes
(d) Given three statements are wrong.
9. Which of the following does not occur, when a rod of Zn metal is dipped in an aqueous solution of CuSO4 ?
(a) blue colour of the oxygen fades gradually. (b) weight of Zn–metal rod decreases.
(c) weight of metal strip of zinc increases.
(d) colour of the surface of Zn road become saffron–red.
10. Which of the following observation obtained, when rod of Cu metal is dipped in an aqueous solution of AgNO3?
(a) No change in the weight of metal rod of Cu occurs.
(b) weight of rod of copper metal decreases
(c) solution become bluish gradually
(d) colour of the surface of rod of Cu metal does not change
11. Which substance get oxidized in the reaction : 2Al + Cr2O3 → Al2O3 + 2Cr ?
(a) Al (b) Cr2O3 (c) Al2O3 (d) Cr
12. Which substance is a reducting agent in the following reaction ?
Reaction : 2Al + Cr2O3→ Al2O3 + 2Cr
(a) Al (b) Cr2O3 (c) Al2O3 (d) Cr
13. In the reaction, 2Na + S→Na2S, which sustance acts as oxidizing agent ?
(a) Na (b) S (c) Na2S (d) None of these.
14. Which of following elements does not possess positive oxidation no. in any of its compound ?
(a) O (b) F (c) Cl (d) I
15. Which of the following oxidation no. does not possess by Cl, Br and I, when they conbines with oxygen forming chemical bond ?
(a) +1 (b) +3 (c) +5 (d) –1
16. Oxygen conbines with which of the element by forming chemical bond, then it possesses positive oxidation no. ?
(a) F (b) Cl (c) Br (d) given all
17. Which of the following element always possesses +1 oxidation state in any of its compound ?
(a) F (b) Ca (c) Cs (d) O
21. Which of the following oxidation no. possesses by oxygen in its compounds ?
(a) –1 (b) +3 (c) –4 (d) +5
22. Which type of metal compounds are nomenclate according to stock notation nomenclature method ?
(a) Metal compound having fixed oxidation no. (b) Comppounds of alkali metals.
(c) Metallic compounds having more than one oxidation no.
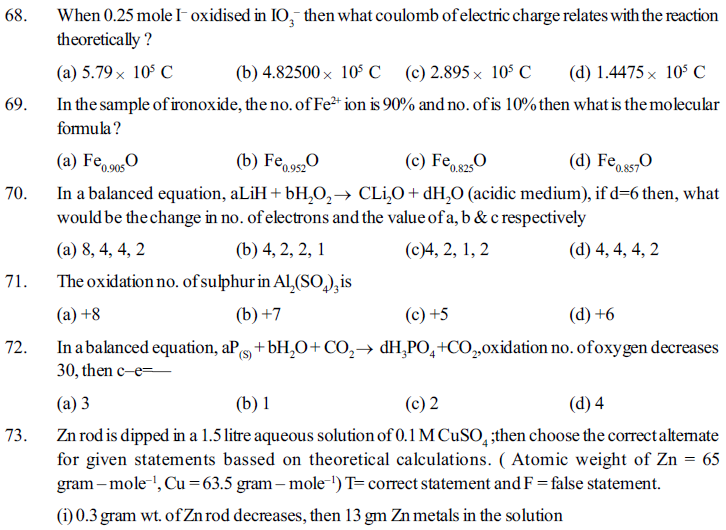
(d) Compounds of non–metal.
23. Molecular fromula of sodium chromate (VI) is _________
(a) Na2Cr2O7 (b) Na2Cr2O4 (c) Na2CrO4 (d) NaCrO4
24. What is the name of K2Cr2O7 according to stock notation nomenclature method ?
(a) Potassium dichromate (VI) (b) Potassium chromate (VI)
(c) Potassium dichromate (III) (d) Potassium dichromate (IV)
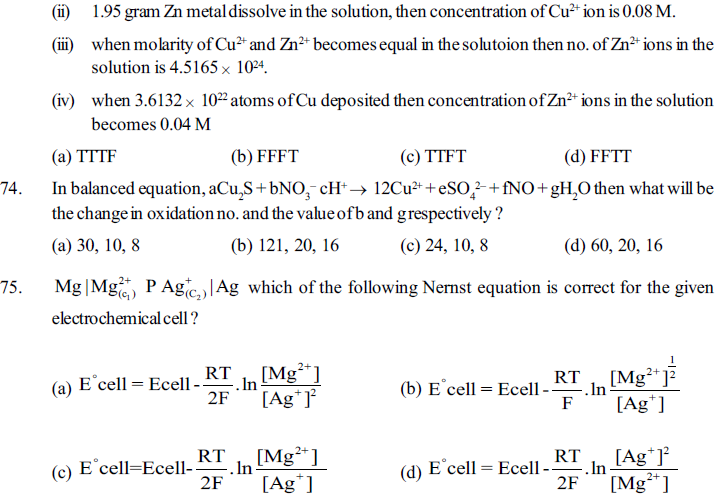
25. What is the name of TiO2, according to stock notation nomenclature method ?
(a) Titanium (II) oxide (b) Titanium oxide (IV)
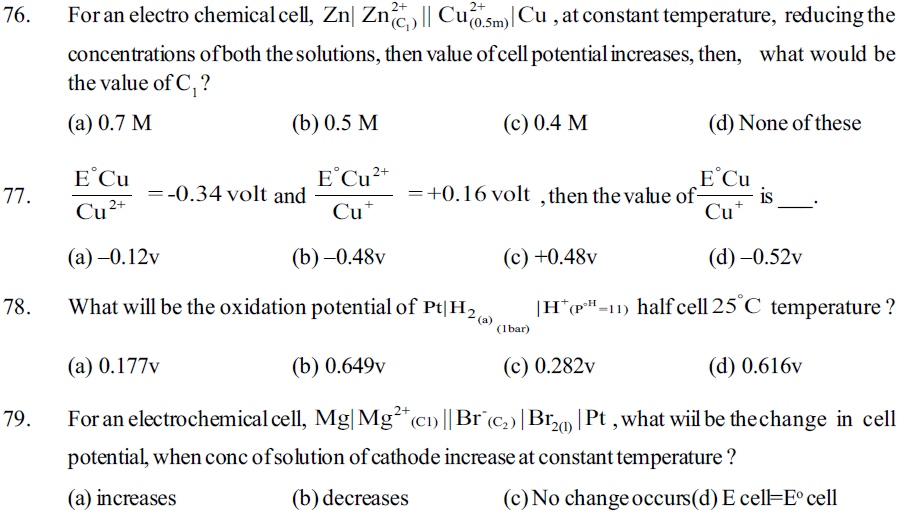
(c) Titanium (IV) oxide (d) Titanium (V) oxide

31. The oxidation no. of sulphur in H2SO3, H2SO4, H2SO5 or sulphuric acid, Caro’s acid (permono sulphuric acid) is respectively________
(a) +4, +6, +7 (b) +3, +6, +6 (c) +4, +6, +6 (d) +5,+6, +6
32. The oxidation no. of sulphur in H2S2O8, H2S2O3, H2S2O7, H2S2O6 or marshal acid (perdisulphuric acid), thiosulphuric acid, oleum, Dithianic acid is respectively
(a) +6, +3, +6, +7 (b) +7, +2, +6, +5 (c) +6, +2, +6, +5 (d) +6. +3, +7, +5
33. What is the oxidation No. of Cr in CrO5, K2Cr2O7, K2CrO4 or in chromium pentoxide , potassium dichromate, potassiam chromate respectively
(a) +4, +5, +6 (b) +5, +6, +6 (c) +4, +6, +6 (d) +6, +6, +6
34. What is the oxidation no. of phosphorus in H3PO3, H3PO4, H3PO2 or phosphorus, acid, Phosphoric acid, phosphinic acid respectively ?
(a) +3, +5, +1 (b) +3, +5, +2 (c) +4, +5, +1 (d) +2, +5, +1
35. What is the oxidation no. of phosphorus in H4P2O7, H5P3O10, (HPO3)3 or pyrophosphoric acid, penta phosphoric acid, triphosphoric acid respectively ?
(a) +4, +5, +3 (b) +6, +5, +5 (c) +5. +5, +5 (d) +4, +5, +5
36. The oxidation no. of chlorine in HClO, HClO2, HClO3, HClO4 or Hypochlorus acid, chloric acid, chloric acid and perchloric acid respectively are
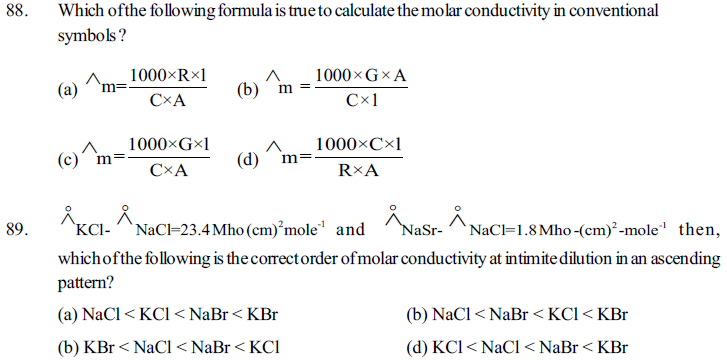
(a) +1, +3, +5, +7 (b)+1, +2, +3, +4 (c) +1, +2, +4, +5 (d) +4, +3, +2, +1
37. What is the oxidation no. of iodine in ICl3, CsI3 respectively ?
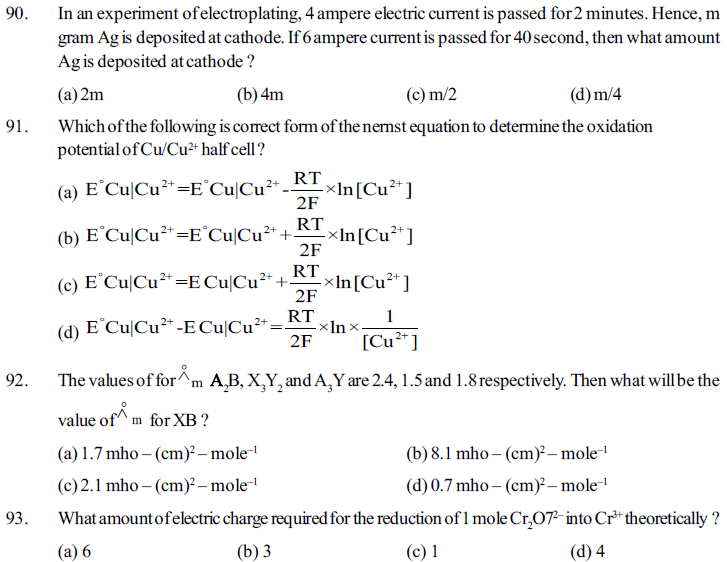
(a) +3, –1 (b) +1, –1 (c) +1/3, –1 (d)+3, –1/3
38. What is the maximum positive oxidation state of halogen element in any of it’s compound ?
(a) +1 (b) +3 (c) +7 (d) +5
39. What is the maximum positive oxidation state of chalcogen element in it’s compound ?
(a) +6 (b) +3 (c) +7 (d) +5
40. What is the the oxidation no. of nitrogen in N3H, H2N2O2, HNO3 or hydrazoic acid, hyponitrons acid, nitrus acid, nitric acid respectively ?
(a) –1, +1, +3, +5 (b) –1/3, +1, +3, +5 (c) +1, +1, +3, +5 (d) +1/3, +2, +2, +5
41. What is the oxidation no. of silicon in zeolite (Na2Al2Si4O12) and tremolite [(Ca2 Mg5(OH)2(Si4O11)2] respectively ?
(a) +4, +3 (b) +4, 4 (c) +2, +2 (d) +3, +4
42. The value of n in AlFxOyn is—
(a) +3 –x–y (b) +3–x–2y (c) +3+x+2y (d) +3+x–y
43. The value of n in AlFXOyn, if x=1 and y=1 ?
(a) +1 (b) +2 (c) 0 (d) +3
44. The value of n in AlFXOyn, if x=2 and y=3 ?
(a) –2 (b) 0 (c) –5 (d) –4
45. What will be the value of x and y respectively in AlFXOy6– ?
(a) 1, 4 (b) 3, 2 (c) 2, 2 (d) 4, 3
46. How many moles of elements are added when 2.5 mole Cr2O72– reduced in Cr3+ ?
(a) 12.5 (b) 15 (c) 7.5 (d) 10
47. What moles of Cr2O72– reduced in Cr3+ by the addition of 12 moles of electrons ?
(a) 6, (b) 5 (c) 2 (d) 12
48. How many mole ferrous (Fe2+) ion oxidized in ferric (Fe3+) ion by the required no. of electrons the oretically to reduced 4 mole Cr2O72– in to Cr3+ ?
(a) 8 (b) 24 (c) 48 (d) 12
49. Theoretically what gram ferrous (Fe2+) ion oxidized in to ferric (Fe3+) ion by passing 2.4125× 105 coulomb electric charge ? (Atomic mass of Fe=56 gram/mole)
(a) 70 gram (b) 140 gram (c) 14 gram (d) 280 gram

80. Aqueous solution of salt of metal B is stored in a vesselof metal A and aqueous solution of solt of metal c can be storedin the vessel of metal B, then, which of the following is the correct descending order of their strength of reducing agent of A, B and c ?
(a) A > B > C (b) A > C > B (c) C > B > A (d) C > A > B
81. Standard oxidation potential of half cells of A/A2+, B/B2+, C/C2+ and D/D2+ are in increasing order, then which of the following statement is correct ?
(a) solution of salt of A2+ can be stored in the vessel of metal B.
(b) solution of salt of D2+ can’t be stored in the vessel of metal C.
(c) solution of salt of D2+ can be stored in the vessel of metal B.
(d) given all three statements are wrong.
82. For which of the following compound, a graph of mular conductivity and (molarity)1/2 is obtained straight line ?
(a) CsCl (b) NH4OH (c) HCOOH (d) given all three
83. Electrolytic cells having molten NaCl, CaCl2 and AlCl3 solutions are connected in series and same electricity is passed then, which of the following ratio of moles of metal obtained at cathode is correct ?
(a) 1:2:3 (b) 3:2:1 (c) 6:2:3 (d) 6:3:2
84. Eored for Fe/Fe2+ and Fe2+/Fe3+ half cells are –0.44volt and +0.77volt respectively then what will be the value of Eoox for Fe/Fe3+ half cell ?
(a) 0.037v (b) 0.33v (c) –0.33v (d) –o.11v
85. 5 faraday electric charge is passed during electrolysis of molten cacl2 solution, then what moles of Ca obtained at cathode experimentally ?
(a) 2.5 mole (b) less then 2.5 mole (c) more then 2.5 mole (d) 5 mole
86. When same electric charge is passed through electrolytic cells containing aqueous solutions of CuSO4, AgNO3 and NiSO4, then what would be prportion of moles of metal obtained at different cathodes respectively ?
(a) 2:1:2 (b) 2:2:1 (c) 1:1:2 (d) 1:2:1
87. What would be the change in PH of the solution, when electrolysis of aqueous solution of CuSO4 is carried out in presence of inert electrodes ?
(a) PH increasses (b) PH decreases (c) no change in PH (d) can’t be predicted
94. What does the potential of the electrochemical cell become zero ?
(a) Eox of anode and Ered of cathode become equal
(b) Eored of anode and Eored of cathode become equal
(c) Ered of anode and Ered of cathode become equal
(d) Concentration of both the half cell become same
95. In an electrochemical cell for which of the following alternative shows Ecell = E°cell ?
(a) k = 1, then... (b) cell reaction is in equilibrium, then
(c) concentration of both the half cells become equal, then
(d) None of these

