Important Points
In this unit, the study of chemical bonding and formation of molecule from the atoms are included. The smallest particle of element is atom and the smallest particle in a compound is molecule. The force or the binding that keeps the atoms in the molecule combined during the formation of molecule is called chemical bonding. The concepts like that of Kossel-Lewis, VSEPR principle, valence bond theory, molecular orbital theory have been presented. In chemical bonding, it has more relation with orbitals around the nucleus and especially the valence orbitals. We do not think about the nucleus but we take into consideration the effect due to its positive charge. Scientists Lewis and Kossel have mentioned the approach of chemical bonding. In this, the atom obtains the octet either by losing or by gaining the electron, which is chemically inert. This is called law of octet. Such bonds are called ionic bonds e.g. NaCl. Also, some atoms share electrons with each other and obtain octet structure resulting into stable covalent molecule. e.g. Cl2. To explain the structures of such molecules he mentioned dot and cross symbols and explained the stability of the molecules. Such a bond is called covalent bond. The approach of Kossel Law is explained in detail in the unit.
When any bond is formed, the distance between their atoms is called bond length and the angle is called bond angle. As you know the bond lengths of single (-) bond, double (=) bond and triple (º) bond are different. The bond angle gives geometrical shapes to molecules viz.180°- linear, 109°28' tetrachedral. You will study in detail about covalent bond which can be of three types. (1) Polar covalent bond in which the electron remains dragged towards the more electronegative atom and +d charge on electropositive atom and -d charge on electronegative atoms are shown. As a result the molecule becomes polar. If the electronegativities of the two atoms are same or the difference between them is less, than non-polar bonds, formed by both the atoms sharing the electrons. In coordinate covalent bond, one of the two atoms sharing a pair of electrons and the second atom completes the octet with the help of this gained electron pair. viz. F3B ¬ NH3. Bond ¬ indicates co-ordinate covalent bond. Over and above, bond length, bond angle, bond enthalpy (bond energy) is also an important concept. Shorter the bond length, more will be the stability and so more energy will be required to break it. Thus, the values of enthalpy may be different according to bond formation. The number of bonds is called bond order which we have studied in detail and also the formula to determine it. Born-Haber showed that the enthalpy evolved in formation of compound is the mathematical results of the enthalpies of several reactions of atoms. It is explained in the unit by discussing the formation of compound like NaCl.
Sometimes, it so happens that the electron pair instead of being localised on any molecule it localises towards other molecule. Thus, the bonds in the molecule can be shown at different positions in the compounds having same molecular formula. Such structures are called resonance structures and energy associated with the changes of these resonance forms is called resonance energy. This can be studied through the molecules of ozone, carbon dioxide, benzene etc.
As we have seen earlier, structures like linear, tetrahedral etc, can be obtained on the basis of bond angle. This study can be used to show the shapes of the molecule by hybridisation of atoms in them, geometrical structures etc. viz. linear BeCl2 - 1800, trigonal BCl3 - 1200, tetrahedral CH4 - 109028'.
Lewis approach being insufficient to explain the shapes of molecules, Sidgwick and Powell proposed one principle which is known as VSEPR principle which was developed by Nyholm and Gillespie and they proposed certain assumptions. In this it is important to note that when non-bonding electron pairs are there, then they show deviation in geometrical structure and bond angle due to repulsion between electron pairs. e.g. Molecule of water has sp3 hybridisation and so its bond angle must be 109028' but it becomes 1040 30' due to repulsion by two non-bonding electron pairs. Hence, it is called distored tetrachedral. The polarity of bond is a vector quantity. Hence, if a polar bond is formed due to difference in electronegativities but another bond of the same type is formed in its opposite direction, then polar bond will be formed but the resultant polarity of the molecules becomes zero and molecule will be non-polar.
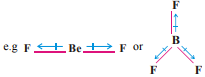
The dipole momentes of polar substances can be calculated for which both the charges +d and -d and the distance between them is to be utilised. More the value of dipole moment, more will be the polar bond and more will be the ionic bond. One important aspect is that polar substances dissolve only in polar solvents and non-polar substances dissolve in nonpolar solvents. e.g. NaCl will dissolve in water. Napthalene will dissolve in benzene. New hypotheses have been presented affter taking into consideration the limitations of the principles for the approach of covalent bond. Two are main from them : (1) Valence Bond Theory and (2) Molecular Orbital Theory. These concepts are based on quantum mechanics. Heitler and London first of all gave the idea of valence bond theory and it was developed by Pauling and Slater.
In the assumptions of valence bond theory the attraction - repulsion forces between positively charged nuclei of two atoms and the electrons arranged in the orbits around them. According to Coulomb's Law if attractive forces are more than repulsive forces then the bond will be formed and molecule will be formed. In this theory, on the basis of the overlapping of valence orbitals different overlaps can be formed. In this type of overlapping the excitation of electrons in valence orbitals can be shown and then formation of molecule by covalent bond with other atoms. viz. In carbon, the electrons of valence orbital 1s2 2s2 2p2 will be excited to give 1s2 2s1 2px1 2py1 2pz1 containing four orbitals with one electron in each and four hydrogen atoms, and hence will give stable molecule like CH4. The geometrical structure, and bond angle can be expressed from the hybridisation associated with it. In such valence bonds, two types of bonds-s and p are also observed. s bond is a covalent bond; it attains axial overlap of internuclear axis. The stability of this bond is more than that of π bond. In the p-bond the axis of the atomic orbitals undergoing overlapping remains parallel to each other and is perpendicular to internuclear axis. π- bonds are less stable in comparison to σ-bonds or they are weaker. Valence bond theory is based on overlapping of valence orbitals. It explains properties like the geometrical shapes, the bond angle etc. very simply but cannot explain magnetic properties.
Scientists Mulliken and Hund suggested molecular orbitals like atomic orbitals and proposed molecular orbital theory. Amongst its important points, the idea that atomic orbitals can also form molecular orbitals was taken into consideration. As many atomic orbitals take part in the formation,
same number of molecular orbitals, their energy, symmetry etc. were taken into consideration. The formation of these types of atomic orbitals can be shown in the formation of homonuclear molecules like H2, Be2, F2 etc. and heteronuclear molecules like CO, NO etc. Molecular orbitals are formed by linear combination of atomic orbitals-LCAO principle. On the basis of these types of combination two types of molecular orbitals are formed which are known as Bonding Molecular Orbitals (BMO) and Anti-Bonding Molecular Orbitals (ABMO). In the formation of rules these types of BMO and ABMO the principles like Hund's rule of maximum spin, Pauli's exclusion principle, Aufbau principle etc. which are applicable in formation of atomic orbital are also obeyed and maintained. In the unit the molecular orbital diagrams of construction of molecular orbitals from the atomic orbitals for formation of homonuclear molecules from H2 to Ne2 elements as well as for formation of heteronuclear molecules like CO, and NO are shown.
M.C.Q.
(1) Which of the following is ionic ?
(a) HCl (b) CHCl3 (c) IF5 (d) KI
(2) When molecule is form by chemical bonding between atoms then
(a) nucleous of combining atoms are participate
(b) valence electrons and inner cell electrons are participate
(c) only valence electrons of combining atoms are participate
(d) only inner cell electrons of combining atoms are participate
(3) Which factor is not responsible for the formation of ionicbond?
(a) crystal lattice energy (b) density
(c) ionisation enthalpy (d) electron gain enthalpy
(4) According to valence-bond theory which magnetic property oxygen possess ?
(a) Paramagnetic (b) Ferrimagnetic (c) Diamagnetic (d) Anti Ferromagnetic
(5) Who was proposed valence-bond theory ?
(a) Mulliken (b) Lenus Pauling (c) Hittler and Londan (d) Hund
(12) Which of the following characteristic is not for covalent compound ?
(a) They do not possesses particular geometical structure
(b) They may be polar or nonpolar
(c) Their boiling and melting point is low
(d) Generally they are insoluble in water
(13) Which of the following possesses ionic and covalent bond ?
(a) CO2 (b) H2SO4 (C) NH4Cl (D) NaI
(14) Whhat is Geometrical Structure of ClF3 molecule ?
(a) Triogonal bipyramid (b) Corn shpae (c) sea-saw (d) T-shape
(15) Which of the following molecule possesses linear structure ?
(a) SO2 (b) CO2 (c) H2O (d) C2 H4
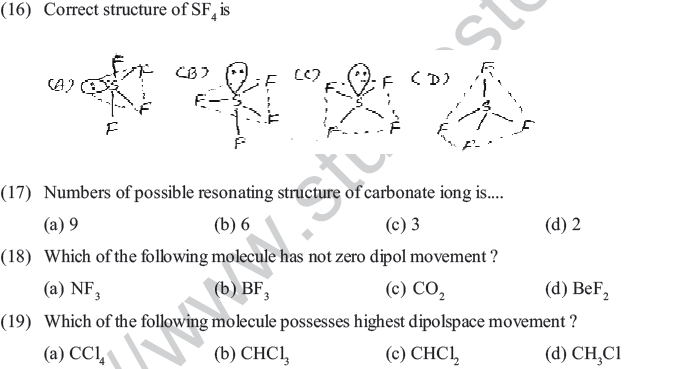
(25) According to VSEPR theory geomety of which block elements can be explain ?
(a) s (b) p (c) d (d) f
(26) Atoms complete octet in valence shell electron during the bond formation. This postulate was proposed by which scientist ?
(a) Powel (b) Lewis (c) Sigdwick (d) Mulliken
(27) Crystal formation is which type of reaction ?
(a) endothermic and exothermic (b) endothermic
(c) exothermic (d) no heat change occurs
(28) Lattice energy of ionic compound depends upon which factor ?
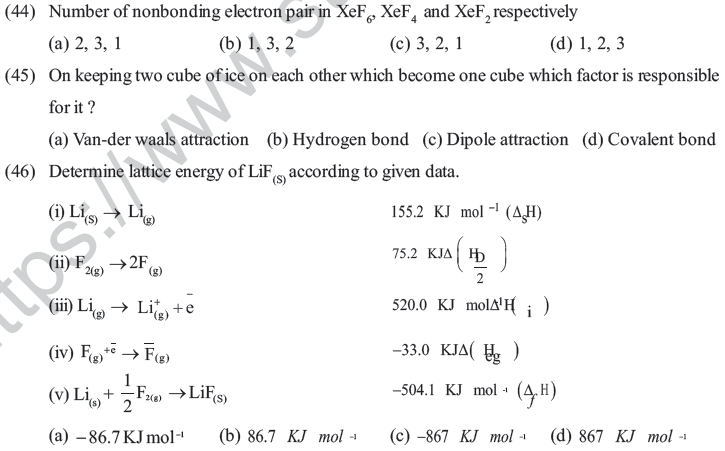
(a) Size of ion (b) Size of ion and charge
(c) charge on ion (d) Arrangement of ion
(32) Which of the following statement is irrelevant for sigma bond ?
(a) strength of sigma bond is not related with overlapping of atomic orbitals.
(b) σ - bond can form by overlapping of S - P orbitals.
(c) σ - bond can form by overlapping of end of atomic orbitals of inner center axis.
(d) This type of overlapping is also known as axial overlaping
(33) In which molecule inter molecular hydrogen bond can be form ?
(a) methanol (b) ethelene glycol (c) p - nitrophenol (d) phenol
(34) In which molecule intra molecular hydrogen bond can be form ?
(a) o - nitro phenol (b) aniline (c) ethylene glycol (d) all of these
(35) Which of the following pair possesses very strong H - bond ?
(a) CH3 COCH3 and CHCl3 (b) HCOOH and CH3 COOH
(c) H2O and H2 (d) SiH4 and SiCl4
(38) In water bond angle is 104o 30 because
(a) Oxygen atom is sp3 hybridised
(b) Repulsion between lone pair election and bonding pair electron
(c) Oxygen has high electronegetivity.
(d) H2O molecule possesses ''V'' - shape.
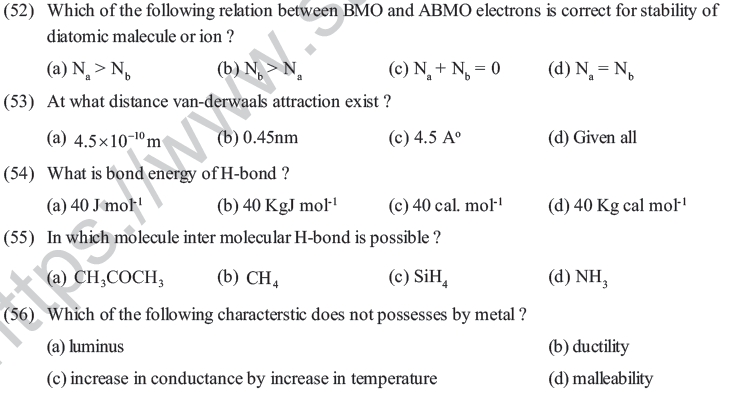
(47) Which of the following statment is incorrect for metallic bond ?
(a) There is attraction between delocalised electrons and atomic karnel
(b) Directionl property is shown by metal
(c) Delocalised electron can change their position easily in crystal
(d) Explanation of metallic bond can be given by 'electron sea model'
(48) Why lattice energy of NaCl > KBr ?
(a) When size of negative ion decrease in ionic crystal then lattice energy increases.
(b) When volume of positive and negative ion is small than then interionic attraction become more and hence latice energy increases.
(c) In ionic crystal when size of positive ion decrease, then lattice energy increases.
(d) All of given
(49) Number of H - bond form by unpaired electrons of liquid NH3 , H2O and HF respectively are
(a) 3, 4, 2 (b) 4, 4, 2 (c) 3, 2, 1 (d) 1, 2, 1
(50) Which of the following pair is not in order for boiling point for 14, 15, 16 and 17 group ?
(a) H2O > H2S (b) HF > HCl (c) CH4 > SiH4 (d) NH3 > PH3
(51) Which of the following compound possesses ionic bond ?
(a) CH4 (b) SiCl4 (c) BF3 (d) MgCl2
(57) On which factor conductance of metals responsible ?
(a) ions (b) delocalised (c) atomic kernel (d) number of atoms
(58) Which of the following figure shows electron-sea model ?
(59) According to which group, hydrogen bond is form in protein molecule present in musecls of living organism ?
(a) -CO- (b) -COOR (c) -CONH- (d) -COOH
(60) On which factor van-der waalls attraction force does not depend ?
(a) numbers of molecules (b) contact surface area of molecules
(c) shape of molecules (d) numbers of electron in molecules
(61) Practicol dipal movement of HCl is 1.03D. If bond length of HCl is 1.275 A° than what will be the pereentage of ionic nature in HCl ?
(a) 7 (b) 17 (c) 43 (d) 21
(62) Which sentence is correct with respect to bond enthalpy ?
(a) As bond order is more, then bond dissociation enthalpy is less
(b) As atomic volume is more, then bond energy is more.
(c) As bond enthalpy is more, then stability of molecule or ion is less.
(d) As number of nonbonding election pair on bonded atom then bond enthalpy is less.
(71) In which of the following molecule double bond possesses two pispace bond ?
(a) S2 (b) O2 (c) C2 (d) H2C = CH2
(72) Mention AB4F2 type molecule.
(a) BrF5 (b) XeF4 (c) SF6 (d) XeOF4
(73) Which of the following is the correct order for lone pair and bonding pair electrons ?
Lp = Lone pair and Bp = Bonding pair
(a) Lp - Lp > Lp - Bp > Bp - Bp (b) Lp - Bp > Lp - Lp > Bp - Bp
(c) Bp - Bp > Lp - Lp > Lp - Bp (d) Lp - Lp > Bp - Bp > Lp - Bp
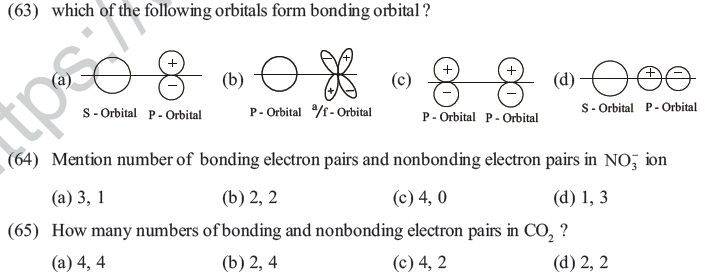
(74) Which theory is useful to determine geometrical structure of molecules ?
(a) molecular orbital theory (b) VSEPR theory
(c) Resonance theory (d) Quantam mechanics
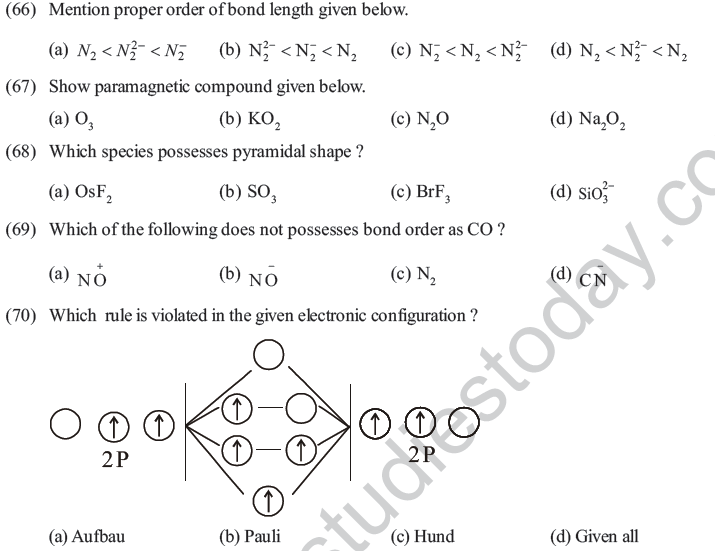
(75) The one outermost electron present in Na element at
(a) one corner of simple cube (b) eight corner of simple cube
(c) center of simple cube (d) each corner of simple cube
(82) NH3 has a higher boiling point than expected because
(a) its density decreases on freezing
(b) with water it forms NH4OH
(c) it has strong inter molecular covalent bonds ?
(d) it has intermolecular hydrogen bonds.
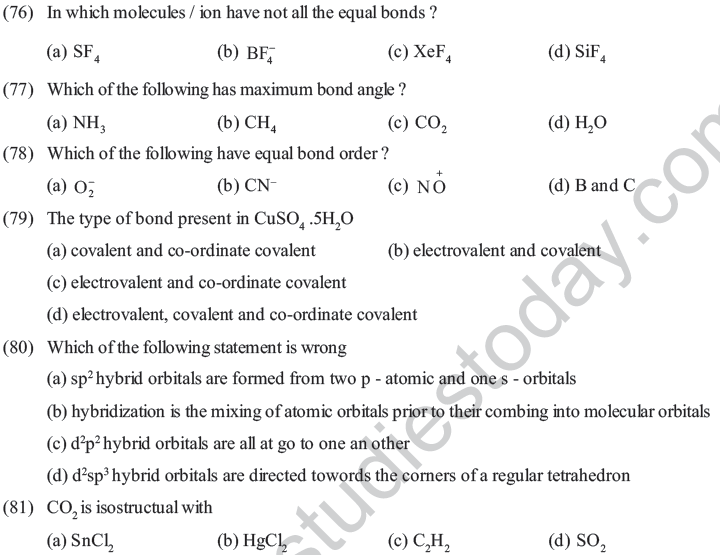
(83) The molecule with zero dipole moment is
(a) chloroform (b) methyl chloride
(c) carbon tetrachloride (d) methylene chloride
(84) Molecular shaper of SF4 , CF4, XeF4 are
(a) the same with 1, 1 and 1 lone pairs of electrons respectively
(b) different with 1, 0 and 2 lone pairs of electrons respectively
(c) different with 0, 1 and 2 lone pairs of electrons respectively
(d) different with 2, 0 and 1 lone pairs of electrons respectively

