Read and download free pdf of CBSE Class 10 Science Periodic classification of elements Sure Shot Questions B. Students and teachers of Class 10 Science can get free advanced study material, revision notes, sure shot questions and answers for Class 10 Science prepared as per the latest syllabus and examination guidelines in your school. Class 10 students should download this study material which will give them more knowledge for all chapters in Science and all important topics which are scoring and can get you more marks. Students should also download free pdf of Chapter wise Notes for Class 10 Science prepared by school teachers as per the latest NCERT, CBSE, KVS books and syllabus issued this year and also download free worksheets and question papers available here to get higher scores in school exams and tests, also click here for more Study Material for Class 10 Science
Study Material for Class 10 Science Chapter 5 Periodic Classification of Elements
Class 10 Science students should refer to the following Pdf for Chapter 5 Periodic Classification of Elements in Class 10. These notes and test paper with questions and answers for Class 10 Science will be very useful for exams and help you to score good marks
Class 10 Science Chapter 5 Periodic Classification of Elements
CBSE Class 10 Science Chapter 5 Periodic classification of elements MCQs
Question. The number of elements in third period of the periodic table is
(a) 2
(b) 8
(c) 10
(d) 14
Answer. B
Question. Study the given table carefully.
| G r o u p s → P e r i o d s ↓ | 1 | 2 | 3 to 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 2 | Q | R | |||||||
| 3 | P | S | T | U |
Identify P, Q, R, T and U.
P Q R T U
(a) Mg O Ne P Ar
(b) O Mg Ar P Ne
(c) Mg O Ar P Ne
(d) O Mg Ne P Ar
Answer. A
Question. The given diagram shows the electron arrangement of two elements, A and B.
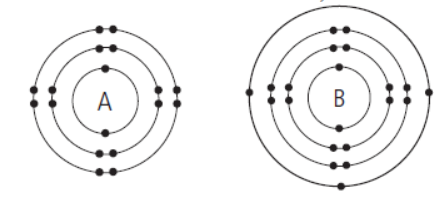
Which of the following statements is correct about the two elements?
(i) A is a Period 3 element.
(ii) B is a Group III element.
(iii) Both A and B have strong metallic characteristics.
(iv) Both A and B from positive ions.
(a) (ii) only
(b) (i) and (ii) only
(c) (iii) and (iv) only
(d) (i), (ii), (iii) and (iv)
Answer. B
Question. The ‘law of octaves’ was enunciated by
(a) Lother Meyer
(b) Mendeleev
(c) Dobereiner
(d) Newlands.
Answer. D
Question. Element X is in period 3 and group III of the periodic table. The electronic configuration of X is
(a) 2, 1
(b) 2, 3
(c) 2, 8, 3
(d) 2, 8, 5
Answer. C
Question. Döberiener’s system of classification into triads was not found to be useful as he could identify only
(a) two triads
(b) three triads
(c) four triads
(d) five triads.
Answer. B
Question. Identify the correct statements.
(i) In Mendeleev’s periodic table, elements were arranged in the order of their increasing atomic masses and it was also observed that there occurs a periodic recurrence of elements with similar physical and chemical properties.
(ii) In 1913, Dmitri Ivnovich Mendeleev showed that the atomic number of an element is more fundamental property than its atomic mass.
(iii) He was the most important contributor to the early development of a Periodic Table of elements wherein the elements were arranged on the basis of their fundamental property, the atomic mass and also on the similarity of chemical properties.
(iv) Among chemical properties, Mendeléev concentrated on the compounds formed by elements with oxygen and hydrogen.
(v) Mendeléev formulated a Periodic Law,which states that ‘the properties of elements are the periodic function of their atomic numbers’.
(vi) Mendeléev’s Periodic Table contains vertical columns called ‘groups’ and horizontal rows called periods.
(a) (ii) and (v)
(b) (i), (iii), (iv), (v) and (vi)
(c) (i), (iii), (iv) and (vi)
(d) All the statements are correct.
Answer. C
Question. Two elements A and B belong to group 1 and 2 respectively. Identify the correct statements.
(i) Valency of A is one while that of B is two.
(ii) Oxide of A has formula AO and that of B is B2O
(iii) Element A is more metallic as compared to element B.
(iv) Element A is smaller than B in size.
(a) (ii) and (iv)
(b) (i) and (iv)
(c) (i) and (iii)
(d) (ii) and (iv)
Answer. C
Question. Considering the elements B, Al, Mg and K, the correct order of their metallic character is
(a) B > Al > Mg > K
(b) Al > Mg > B > K
(c) Mg > Al > K > B
(d) K > Mg > Al > B
Answer. D
Question. State True (T) or False (F) for the given statements.
(i) John Newlands, arranged the known elements in the order of increasing atomic numbers.
(ii) He started with the element having the lowest atomic mass (hydrogen) and ended at thorium which was the 56th element.
(iii) He found that every ninth element had properties similar to that of the first.
(iv) The properties of lithium and sodium were found to be the same.
(v) Beryllium and magnesium resemble each other.
(vi) Newlands’ law of octaves worked well with higher elements only.
(i) (ii) (iii) (iv) (v) (vi)
(a) F T F T T T
(b) T F T F T T
(c) T F F F T T
(d) F T F T T F
Answer. D
Question. An element X belongs to group 14th and 2nd period of the periodic table. Its atomic number will be
(a) 6
(b) 14
(c) 7
(d) 15
Answer. A
Question. Which of the following pairs of atomic numbers represents elements belonging to the same group?
(a) 11 and 20
(b) 12 and 30
(c) 13 and 31
(d) 14 and 33
Answer. C
Question. Moving down a group of the periodic table,
(a) the number of valence electrons increases
(b) the metallic characteristics of the elements increases
(c) the ability of the elements to form positive ions decreases
(d) the atomic number of the elements decreases.
Answer. B
Question. The number of elements in the 5th period of periodic table are
(a) 8
(b) 18
(c) 10
(d) 32
Answer. B
Question. The basic character of MgO, SrO, K2O and NiO increases in the order
(a) K2O < SrO < MgO < NiO
(b) NiO < MgO < SrO < K2O
(c) MgO < NiO < SrO < K2O
(d) K2O < MgO < NiO < SrO
Answer. B
Question. X, Y and Z are the three elements, each one belongs to any one of the groups IA, IIIA and VA. The oxide of X is amphoteric, the oxide of Y is highly acidic, and the oxide of Z is highly basic. Identify the groups to which these elements X, Y and Z belong?
X Y Z
(a) VA IA IIIA
(b) IA VA IIIA
(c) IIIA IA VA
(d) IIIA VA IA
Answer. D
Question. Moving across a period of the Periodic Table,
(a) the elements become more electronegative
(b) the metallic character of the elements increases.
(c) the ability of the elements to lose electrons increases.
(d) the elements form ions with increasing negative charge.
Answer. A
Question. The proton number of four elements are given below :
| Element | W | X | Y | Z |
| Proton number | 8 | 13 | 15 | 19 |
Which of the following pairs of elements belong to the same period of the periodic table?
(a) W and X
(b) W and Y
(c) X and Y
(d) Y and Z
Answer. C
Question. Which element has the largest size in the second period?
(a) N
(b) F
(c) Li
(d) Be
Answer. C
Question. From the given set of metals and non-metals identify the non-metals. S, Mg, Al, P, N, Na, K.
(a) S, P, K
(b) Mg, Al, Na
(c) S, P, N
(d) S, Al, K
Answer. C
Question. Which of the following combination of elements belong to the same group?
(a) N, P, As
(b) Li, Be, Al
(c) Na, Mg, Al
(d) O, S, Cl
Answer. A
Question. From top to bottom in a group of the periodic table the electropositive character of the element
(a) increases
(b) decreases
(c) remains unchanged
(d) changes irregularly.
Answer. A
Question. In the third period of the periodic table, the element having smallest size is
(a) Na
(b) Al
(c) Cl
(d) Si
Answer. C
Question. Which is true about electronegativity order?
(a) P > Si
(b) C > N
(c) Br > Cl
(d) Sr > Ca
Answer. A
Question. In the given metals one with the smallest size is
(a) Rb
(b) Cs
(c) K
(d) Na
Answer. D
Question. Which of the following is the most nonmetallic element?
(a) Br
(b) Cl
(c) P
(d) S
Answer. B
Question. Which of the following elements is a metalloid?
(a) Pb
(b) Sb
(c) Bi
(d) Zn
Answer. B
Question. Identify the pair of atomic numbers representing s-block elements.
(a) 7, 15
(b) 9, 17
(c) 2, 10
(d) 11, 12
Answer. D
Question. Which of the given pairs of atomic numbers represents elements in the same group?
(a) 11, 19
(b) 6, 12
(c) 4, 16
(d) 8, 17
Answer. A
Question. The effective nuclear charge acting on the valence shell electrons
(a) increases across a period
(b) decreases down the group
(c) both (a) and (b)
(d) none of the above.
Answer. C
Question. Which of the following will have equal number of electrons?
(a) Cl– and Br–
(b) Na+ and Mg2+
(c) Ar and Ne
(d) Mg2+ and Ca2+
Answer. B
CBSE Class 10 Science Chapter 5 Periodic classification of elements Case Based MCQs
Case I : Read the passage given below and answer the following questions.
Maximum number of electrons that can be accommodated in a shell is given by the formula : 2n2, where n is the number of the outermost from the nucleus.
For example, K shell – 2 × (1)2 ⇒ 2, hence, K-shell can accommodate maximum 2 electrons.L shell – 2 × (2)2 ⇒ 8, hence, L-shell can accommodate maximum 8 electrons. In the modern periodic table, elements are placed according to their electronic configuration. The elements present in any group have the same number of valence electrons. The elements present in any period contain the same number of shells. The first period of the modern periodic table corresponds to the filling of electrons in the first energy shell, i.e., K-shell, first period has two elements. The second period of the periodic table corresponds to the filling of electrons in the second energy shell, i.e., L-shell, second period contains eight elements. The third, fourth, fifth, sixth and seventh periods have 8, 18, 18, 32 and 32 elements respectively.
Question. Electronic configuration of an element ‘X ’ is 2, 1. The number of elements present in the period to which ‘X ’ belongs is
(a) 8
(b) 32
(c) 18
(d) 2
Answer. A
Question. Among the given elements A, B, C, D and E with atomic numbers 2, 3, 7, 10 and 30 respectively, which of these belong to the same period?
(a) A, B, C
(b) B, C, D
(c) A, D, E
(d) B, D, E
Answer. B
Question. The elements A, B, C and D have atomic numbers 4, 12, 17 and 19 respectively. Which pair of elements belong to the same period?
(a) B and C
(b) A and B
(c) A and D
(d) C and D
Answer. A
Question. Which of the following have the same number of electrons in outermost shell?
(a) Elements with atomic numbers 3, 11, 19
(b) Elements with atomic numbers 14, 15, 16
(c) Elements with atomic numbers 12, 20, 28
(d) Elements with atomic numbers 10, 18, 26
Answer. A
Question. Which of the following elements has two shells and both are completely filled?
(a) Helium
(b) Neon
(c) Calcium
(d) Fluorine
Answer. B
Case II : Read the passage given below and answer the following questions.
Mendeleev arranged the 63 elements known at that time in order of their ascending atomic masses and prepared a periodic table. Mendeleev’s periodic table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’. Elements with similar properties were placed in same groups.
The basis of Mendeleev’s classification is his periodic law which states that :
(I) Atomic mass is the fundamental property of elements.
(II) The physical and chemical properties of elements are periodic function of their atomic masses.
Question. Which of the following metals is not placed in eighth group of Mendeleev’s periodic table?
(a) Fe
(b) Na
(c) Co
(d) Ni
Answer. B
Question. In Mendeleev’s periodic table, silver belongs to IB group. The group to which silver belongs in the modern periodic table is
(a) first
(b) eleventh
(c) tenth
(d) sixteenth.
Answer. B
Question. In Mendeleev’s periodic table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the periodic table later?
(a) Chlorine
(b) Silicon
(c) Oxygen
(d) Germanium
Answer. D
Question. The properties of eka-aluminium predicted by Mendeleev were the same as properties of which element that was discovered later?
(a) Scandium
(b) Germanium
(c) Gallium
(d) Aluminium
Answer. C
Question. Which of the following statements is not correct about Mendeleev’s periodic table?
(a) In the Mendeleev’s periodic table, some places were left vacant for new elements which were not discovered at that time.
(b) Group VIII like groups I-VII has been divided into two sub-groups A and B.
(c) The group of an element in the periodic table represents its valency.
(d) Li and C belong to same period in Mendeleev’s periodic table.
Answer. B
Case III : Read the passage given below and answer the following questions.
Study the following table in which positions of six elements A, B, C, D, E and F are shown as they are in the modern periodic table :
| Group → Period ↓ | 1 | 2 | 3-12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 2 | A | B | C | ||||||
| 3 | D | E | F |
Question. Which element in the given table has same number of electrons as in K+ and Cl–?
(a) C
(b) F
(c) E
(d) D
Answer. B
Question. The formula of the oxide of element D will be
(a) DO
(b) D2O
(c) D2O3
(d) D2O5
Answer. C
Question. Which of the following elements has most metallic character?
(a) F
(b) D
(c) E
(d) B
Answer. B
Question. Element E forms a chloride with formula
(a) E Cl2
(b) E Cl3
(c) E Cl4
(d) ECl
Answer. C
Question. Which of the following elements is a metalloid?
(a) A
(b) B
(c) C
(d) E
Answer. D
PERIODIC CLASSIFICATION OF ELEMENTS
1. Upto which element, the Law of Octaves was found to be applicable.
(a) Oxygen
(b) Calcium
(c) Cobalt
(d) Potassium
2. According to Mendeleev's Periodic Law, the elements were arranged in the periodic table in the order of
(a) increasing atomic number
(b) decreasing atomic number
(c) increasing atomic masses
(d) decreasing atomic masses
3. In Mendeleev ’s Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the perioidc table later
(a) Germanium
(b) Chlorine
(c) Oxygen
(d) Silicon
4. Which of the following statement (s) about the Modern Periodic Table are incorrect.
(i) The elements in the Modern Periodic Table are arranged on the basis
of their decreasing atomic number
(ii) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic masses
(iii) Isotopes are placed in adjoining group (s) in the Periodic Table
(iv) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic number
(a) (i) only (b) (i), (ii) and (iii)
(c) (i), (ii) and (iv) (d) (iv) only
5. Which of the following statements about the Modern Periodic Table is correct:
(a) It has 18 horizontal rows known as Periods
(b) It has 7 vertical columns known as Periods
(c) It has 18 vertical columns known as Groups
(d) It has 7 horizontal rows known as Groups
6. Which of the given elements A, B, C, D and E with atomic number 2, 3, 7, 10 and 30 respectively belong to the same period?
(a) A, B, C
(b) B, C, D
(c) A, D, E
(d) B, D, E
7. The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively. Which pair of elements belong to the same group?
(a) A and B
(b) B and D
(c) A and C
(d) D and E
8. Where would you locate the element with electronic configuration 2,8 in the Modern Periodic Table?
(a) Group 8
(b) Group 2
(c) Group 18
(d) Group 10
9. An element which is an essential constituent of all organic compounds belongs to
(a) group 1
(b) group 14
(c) group 15
(d) group 16
10. Which of the following is the outermost shell for elements of period 2?
(a) K shell
(b) L shell
(c) M shell
(d) N shell
11. Which one of the following elements exhibit maximum number of valence electrons?
(a) Na
b) Al
(c) Si
(d) P
12. Which of the following gives the correct increasing order of the atomic radii of O, F and N ?
(a) O, F, N
(b) N, F, O
(c) O, N, F
(d) F, O, N
13. Which among the following elements has the largest atomic radii?
(a) Na
(b) Mg
(c) K
(d) Ca
14. Which of the following elements would lose an electron easily?
(a) Mg
(b) Na
(c) K
(d) Ca
15. Which of the following elements does not lose an electron easily?
(a) Na
(b) F
(c) Mg
(d) Al
16. Which of the following are the characteristics of isotopes of an element?
(i) Isotopes of an element have same atomic masses
(ii) Isotopes of an element have same atomic number
(iii) Isotopes of an element show same physical properties
(iv) Isotopes of an element show same chemical properties
(a) (i), (iii) and (iv) (b) (ii), (iii) and (iv)
(c) (ii) and (iii) (d) (ii) and (iv)
17. Arrange the following elements in the order of their decreasing metallic character Na, Si, Cl, Mg, Al
(a) Cl > Si >Al > Mg >Na
(b) Na >Mg >Al >Si > Cl
(c) Na > Al > Mg > Cl > Si
(d) Al > Na> Si > Ca> Mg
18. Arrange the following elements in the order of their increasing nonmetallic character Li, O, C, Be, F
(a) F < O < C < Be < Li
(b) Li < Be < C < O< F
(c) F < O < C < Be < Li
(d) F < O < Be < C < Li
19. What type of oxide would Eka– aluminium form?
(a) EO3
(b) E3O2
(c) E2O3
(d) EO
20. Three elements B, Si and Ge are
(a) metals
(b) non-metals
(c) metalloids
(d) metal, non-metal and metalloid respectively
21. Which of the following elements will form an acidic oxide?
(a) An element with atomic number 7
(b) An element with atomic number 3
(c) An element with atomic number 12
(d) An element with atomic number 19
22. The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element belong?
(a) Metal
(b) Metalloid
(c) Non-metal
(d) Left-hand side element
23. Which one of the following does not increase while moving down the group of the periodic table?
(a) Atomic radius
(b) Metallic character
(c) Valence
(d) Number of shells in an element
24. On moving from left to right in a period in the periodic table, the size of the atom.
(a) increases
(b) decreases
(c) does not change appreciably
(d) first decreases and then increases
25. Which of the following set of elements is written in order of their increasing metallic character?
(a) Be Mg Ca
(b) Na Li K
(c) Mg Al Si
(d) C O N
26. The three elements A, B and C with similar properties have atomic masses X, Y and Z respectively. The mass of Y is approximately equal to the average mass of X and Z. What is such an arrangement of elements called as? Give one example of such a set of elements.
27. Elements have been arranged in the following sequence on the basis of their increasingatomic masses. F, Na, Mg, Al, Si, P, S, Cl, Ar, K
(a) Pick two sets of elements which have similar properties.
(b) The given sequence represents which law of classification of elements?
28. Can the following groups of elements be classified as Dobereiner's triad ?
(a) Na, Si, Cl (b) Be, Mg, Ca
Atomic mass of Be 9; Na 23; Mg 24; Si 28; Cl 35; Ca 40
Explain by giving reason.
29. In Mendeleev ’s Periodic Table the elements were arranged in the increasing order of their atomic masses. However, cobalt with atomic mass of 58.93 amu was placed before nickel having an atomic mass of 58.71 amu. Give reason for the same.
30. “Hydrogen occupies a unique position in Modern Periodic Table”. Justify the statement.
31. Write the formulae of chlorides of Eka-silicon and Eka-aluminium, the elements predicted by Mendeleev.
32. Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell. Give the group number to which they belong in the Modern Periodic Table. Also, give their valencies.
33. If an element X is placed in group 14, what will be the formula and the nature of bonding of its chloride?
34. Compare the radii of two species X and Y. Give reasons for your answer.
(a) X has 12 protons and 12 electrons
(b) Y has 12 protons and 10 electrons
35. Arrange the following elements in increasing order of their atomic radii.
(a) Li, Be, F, N (b) Cl, At, Br I
36. Identify and name the metals out of the following elements whose electronic configurations are given below.
(a) 2, 8, 2 (b) 2, 8, 1
(c) 2, 8, 7 (d) 2, 1
37. Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
38. Arrange the following elements in the increasing order of their metallic character Mg, Ca, K, Ge, Ga
39. Identify the elements with the following property and arrange them in increasing order of their reactivity
(a) An element which is a soft and reactive metal
(b) The metal which is an important constituent of limestone
(c) The metal which exists in liquid state at room temperature
40. Properties of the elements are given below. Where would you locate the following elements in the periodic table?
(a) A soft metal stored under kerosene
(b) An element with variable (more than one) valency stored under water.
(c) An element which is tetravalent and forms the basis of organic chemistry
(d) An element which is an inert gas with atomic number 2
(e) An element whose thin oxide layer is used to make other elements corrosion resistant by the process of “ anodising”
41. An element is placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration
(c) Write the balanced equation when it burns in the presence of air
(d) Write a balanced equation when this oxide is dissolved in water
(e) Draw the electron dot structure for the formation of this oxide
42. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s)
(c) What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed
(d) Draw the electron dot structure of the divalent halide
43. Atomic number of a few elements are given below 10, 20, 7, 14
(a) Identify the elements
(b) Identify the Group number of these elements in the Periodic Table
(c) Identify the Periods of these elements in the Periodic Table
(d) What would be the electronic configuration for each of these elements?
(e) Determine the valency of these elements
44. In which form matter is present around us?
45. At present, how many elements are known to us?
46. The earliest attempt in classifying elements resulted in the formation of two groups of elements. What are they?
47. Who made the first attempt of classifying elements?
48. On what basis Dobereiner classified elements?
49. Dobereiner classified elements into how many groups?
50. What name was given to Dobereiner groups?
51. What is the total number of elements in Dobereiner groups?
52. How did John Newlands classify elements?
53. Name the first element of Newland’s octaves.
54. Name the last element of Newland’s octaves.
55. What is your observation from Newland’s octaves?
56. What is Newland’s Law of octaves?
57. Besides atomic masses, on what other basis were the elements arranged in the Mendleev’s periodic table?
58. Which chemical property of an element was treated as one of the basic property for classifying elements and why?
59. What name is given to vertical columns in Mendleev’s periodic table?
60. What name is given to horizontal rows in Mendleev’s periodic table?
61. While developing the Periodic table, at few places Mendleev inverted the sequence of some elements i.e. he placed an element with slightly greater atomic mass before the element of lower atomic mass. Why did he do so?
62. Though the atomic mass of cobalt (58.9) is greater than nickel (58.7) yet Co is placed before Ni in Mendleev’s periodic table. Why?
63. Which elements did not exist at the time of Mendleev’s periodic classification? What name was given to these elements?
64. In what way hydrogen resembles alkali metals?
65. In what way hydrogen resembles halogens?
66. Why hydrogen cannot be given a fixed position in periodic table?
67. What is the first limitation of Mendleev’s periodic table?
68. How isotopes of all the elements posed a challenge to Mendleev’s periodic table?
69. Who proposed that atomic number is the more fundamental property for classifyingelements?
70. In Modern periodic table, How do elements belonging to the same group resemble each other? Write two points.
71. Different elements have same number of shells, in group or in period?
72. First period of the Modern periodic table contains only two elements. Justify.
73. How many elements are present in second group of the periodic table? Justify.
74. “The valence electrons determine the kind and number of bonds formed by an element”. Justify.
Please click the link below to download CBSE Class 10 Science Periodic classification of elements Sure Shot Questions B.
| CBSE Class 10 Science Electricity |
| CBSE Class 10 Science Electricity Notes |
| CBSE Class 10 Science Electricity Sure Shot Questions A |
| Class 10 Science Electricity Exam Notes |
CBSE Class 10 Science Chapter 5 Periodic Classification of Elements Study Material
We hope students liked the above Study Material for Chapter 5 Periodic Classification of Elements designed as per the latest syllabus for Class 10 Science released by CBSE. Students of Class 10 should download the Study Material in Pdf format, read the notes and related questions and solutions given in above Class 10 Science Study Material on daily basis. All latest Study Material have been developed for Science by referring to the most important and regularly asked topics which the students should learn and practice to get better score in school tests and examinations. Expert teachers of studiestoday have referred to NCERT book for Class 10 Science to develop the Science Class 10 Study Material. After solving the questions given in the Study Material which have been developed as per latest course books also refer to the NCERT solutions for Class 10 Science designed by our teachers. Also download Class 10 Science Sample Papers given on studiestoday. After solving these you should also refer to Class 10 Science MCQ Test for the same chapter.
You can download free study material for Class 10 Science Chapter 5 Periodic Classification of Elements for latest academic session from StudiesToday.com
Yes, the study material given here for Class 10 Science Chapter 5 Periodic Classification of Elements is for current CBSE session
All study maetrial for CBSE Class 10 Science Chapter 5 Periodic Classification of Elements is free

