Read and download free pdf of CBSE Class 10 Science Periodic classification of elements Notes. Students and teachers of Class 10 Science can get free advanced study material, revision notes, sure shot questions and answers for Class 10 Science prepared as per the latest syllabus and examination guidelines in your school. Class 10 students should download this study material which will give them more knowledge for all chapters in Science and all important topics which are scoring and can get you more marks. Students should also download free pdf of Chapter wise Notes for Class 10 Science prepared by school teachers as per the latest NCERT, CBSE, KVS books and syllabus issued this year and also download free worksheets and question papers available here to get higher scores in school exams and tests, also click here for more Study Material for Class 10 Science
Study Material for Class 10 Science Chapter 5 Periodic Classification of Elements
Class 10 Science students should refer to the following Pdf for Chapter 5 Periodic Classification of Elements in Class 10. These notes and test paper with questions and answers for Class 10 Science will be very useful for exams and help you to score good marks
Class 10 Science Chapter 5 Periodic Classification of Elements
In the beginning of 18th century Joseph Louis Proust stated that hydrogen atom is the building material and atoms of all other elements are simply due to the combination of number of hydrogen atoms. (It is to be noted that at his time the atomic weight of all elements were given as whole numbers and the atomic weight of hydrogen was taken as one.)
DOBEREINER’S TRIADS
A German chemist Johann Wolfgang Dobereiner (1829) noted that there were groups of elements with three elements known as triads. Elements in each group or a triad possess with similar chemical properties. Dobereiner discovered that “the relative atomic mass of the middle element in each triad was close to the average of the relative atomic masses of the other two elements”. This statement is called the Dobereiner’s law of Triads.
- In this table, atomic mass of sodium is equal to arithmetic mean of atomic masses of lihtium and potassium. Similarly, atomic mass of strontium is equal to arithmetic mean of atomic masses of calcium and barium.
LIMITATION OF DOBEREINER’S TRIADS:
- All the then known elements could not be arranged in the form of triads.
- The law failed for very low mass or for very high mass elements. In case of F, Cl, Br, the atomic mass of Cl is not an arithmetic mean of * As the techniques improved for measuring atomic masses accurately, the law was unable to remain strictly valid.
NEWLANDS’ LAW OF OCTAVES
Newlands law of octaves states that when elements are arranged in the ascending order of their atomic masses they fall into a pattern in which their properties repeat at regular intervals. Every eighth element starting from a given elements resembles in its properties to that of the starting element.
LIMITATION OF NEWLANDS’ OCTAVES:
- Newlands’ Octaves could be valid upto calcium only; as beyond calcium, elements do not obey the rules of Octaves.
- Newlands’ Octaves was valid for lighter elements only.
- It appears that Newlands did not expect the discovery of more elements than 56 which were discovered till his time
- More than one element had to be placed in some of the groups; in order to place the elements having similar properties in one group. But in order to do so, he also put some dissimilar elements in same group.
- Iron; which has similar property as cobalt and nickel, was placed far from them.
- Cobalt and nickel were placed in the group with chlorine and fluorine in spite of having different properties.
- In spite of above limitations; Newlands was the first scientist who arranged the elements in order of their increasing relative atomic masses.
Question. Did Dobereiner’s triads also exist in the columns of Newlands’ Octaves? Compare and find out.
Answer. Yes. Lithium, sodium and potassium; beryllium; magnesium and calcium are two triads that also exist in the columns of Newland’s octaves.
Question. What were the limitations of Dِ bereiner’s classification?
Answer. Please see above notes
Question. What were the limitations of Newlands’ Law of Octaves?
Answer. Please see above notes
MENDELEEV’S PERIODIC TABLE
Mendeleef arranged the elements known at that time in a chart in a systematic order in the increasing order of their atomic weights. He divided the chart into 8 vertical columns known as groups. Each group is divided into A, B sub groups. Each column contained elements of similar chemical properties.
The elements in the first column, for example, react with oxygen to form compounds with the general formula R2O. For example, Li, Na and K when react with oxygen form compounds like Li2O, Na2O and K2O respectively.
Elements of the second column react with oxygen to form compounds with the general formula RO. For example, Be, Mg and Ca when react with oxygen form BeO, MgO and CaO.
Mendeleef tried to explain the similarities of elements in the same group in terms of their common valency.
THE PERIODIC LAW:
Based on Mendeleeff’s observations regarding the properties of elements in the periodic table, a law known as the periodic law of the properties of elements was proposed.
“The law states that the physical and chemical properties of the elements are a periodic function of their atomic weights.”
SALIENT FEATURES AND ACHIEVEMENTS OF THE MENDELEEFF’S PERIODIC TABLE:
1. Groups and sub-groups: There are eight vertical columns in Mendeleeff’s periodic table called as groups. They are represented by Roman numerals I to VIII. Elements present in a given vertical column (group) have similar properties. Each group is divided into two subgroup‘A’ and ‘B’. The elements within any sub-group resemble each other to great extent. For example, sub-group IA elements called ‘ ali metals’ (Li, Na, K, Rb, Cs, Fr) resemble each other very much.
2. Periods: The horizontal rows in Mendeleeff’s periodic table are called periods. There are seven periods in the table, which are denoted by Arabic numerals 1 to 7. A period comprises the entire range of elements after which properties repeat themselves.
3. Predicting the properties of missing elements: Based on the arrangement of the elements in the table he predicted that some elements were missing and left blank spaces at the appropriate places in the table.
Mendeleef believed that some new elements would be discovered definitely. He predicted the properties of these new additional elements in advance purely
depending on his table. His predicted properties were almost the same as the observed properties of those elements after their discovery. He named those elements tentatively by adding the prefix ‘eka’ (eka is a Sanskrit numeral means one) to the name of the element immediately above each empty space. The predicted the properties of elements namely eka-aluminium, eka-boron, eka-aluminium and ekasiliconwere close to the observed properties of Scandium, Gallium and Germanium respectively which were discovered later.
4. Correction of atomic mass: the correct placement of elements in Mendeleeff’s periodic table helped in correcting the atomic masses of some elements like, Beryllium, Indium, Gold.
For example, At the time of Mendeleef, beryllium was given atomic weight 13.5. Atomic weight = equivalent weight × valency
The equivalent weight of Be was found experimentally as 4.5 and its valency was understood as 3. Therefore atomic weight of beryllium was given as 4.5 × 3 = 13.5. With this atomic weight it had to be placed in a wrong group in the table. He said that its valency should be only 2 and then its atomic weight then would be 4.5 × 2 = 9. If atomic weight of ‘Be’ is 9 it would fit in the second group and its properties practically are similar to Mg, Ca etc., of the second group elements. He also helped in the calculation of the correct atomic weights of ‘Indium’ and ‘Gold’ in this
manner.
5. Some anomalous series of elements like ‘Te’ and ‘I’ were observed in the table. The anomalous series contained element with more atomic weight like ‘Te’ (127.6 u) placed before the element with less atomic weight like ‘I’ (126.9 u). Mendeleeff accepted minor inversions in the order of increasing atomic weight when these inversions resulted in elements being placed in the correct groups.It was the extraordinary thinking of Mendeleeff that made the chemists to accept the periodic table and recognise Mendeleeff more than anyone else as the originator of the periodic law.
LIMITATIONS OF MENDELEEFF’S PERIODIC TABLE:
1. Position of hydrogen: The position of hydrogen in the table is not certain because it can be placed in group IA as well as in group VIIA as it resembles both with alkali metals of IA group and halogens of VIIA group.
2. Anomalous pair of elements: Certain elements of highest atomic mass precede those with lower atomic mass. For example, tellurium (atomic mass 127.6) precedes iodine (atomic mass 126.9). Cobalt and nickel: argon and potassium which were placed in table by deviating the basis of classification (placement in ascending order of atomic masses). For example, potassium (atomic mass 39) placed after argon (atomic mass 40). Similar situation was found in pairs of cobalt and nickel and tellurium, iodine.
3. Dissimilar elements placed together: elements with dissimilar properties were placed in same group as sub-group A and sub-group B. For example, alkali metal like Li, Na, K etc., of IA group have little resemblance with coinage metals like Cu, Ag, Au of IB group.
4. Some similar elements separated: some similar elements like ‘copper and mercury’ and ‘silicon and thalium’ etc are placed in different groups of the periodic table.
5. Position of isotopes: isotopes of elements are placed in the same position in the table.
Question. Use Mendeléev’s Periodic Table to predict the formulae for the oxides of the following elements:
K, C, AI, Si, Ba.
Answer. Oxygen is a member of group VIA in Mendeleef’s Periodic Table. Its valency is 2. Similarly,the valencies of all the elements listed can be predicted from their respective groups. This can help in writing the formulae of their oxides.
(i) Potassium (K) is a member of group IA. Its valency is 1. Therefore , the formula of its oxide is K2O .
(ii) Carbon (C) is a member of group IVA. Its valency is 4. Therefore, the formula of its oxide is C2O4 or CO2.
(iii) Aluminium (Al) belongs to groups IIIA and its valency is 3. The formula of the oxide of the element is Al2O3.
(iv) Silicon (Si) is present in group IVA after carbon. Its valency is also 4. The formula of its oxide is Si2O4 or SiO2.
(v) Barium (Ba) belongs to group IIA and the valency of the element is 2. The formula of the oxide of the element is Ba2O2 or BaO.
Question. Besides gallium, which other elements have since been discovered that were left by Mendeléev in his Periodic Table? (any two)
Answer. Scandium and germanium are the two elements that had been left by Mendeleef.
Question. What were the criteria used by Mendeléev in creating his Periodic Table?
Answer. The criteria used by Mendeleef were
(i) Physical and chemical properties of the elements.
(ii) Atomic masses in increasing order.
Question. Why do you think the noble gases are placed in a separate group?
Answer. Noble gases are also called inert gases because they have a complete octet and hence, are very stable. They do not react with other elements due to their stability. Since they all are unreactive, have complete octet and similar behaviour so they are placed in a separate group .
THE MODERN PERIODIC TABLE
Based on the modern periodic law, a number of forms of periodic table have been proposed from time to time but general plan of the table remained the same as proposed by Mendeleev.The table which is most commonly used and which is based upon the electronic configuration of elements is called the long form of the periodic table. This is called the modern periodic table. Long form of the periodic table is a chart of elements in which the elements have been
arranged in the increasing order of their atomic numbers. This table consists of horizontal rows called periods and vertical columns called groups.
♦ The modern periodic table has also been divided into four blocks known as s,p,d and f blocks.
CBSE Class 10 Science Chapter 5 Periodic classification of elements Very Short Answer Type Questions
Question. What is the valency of silicon with atomic number 14?
Answer. Atomic number of silicon = 14
Electronic configuration = 2, 8, 4
As silicon (Si) contains four electrons in its outermost shell, its valency will be four.
Question. Write any one difference in the electronic configurations of group 1 and group 2 elements.
Answer. Group 1 elements have one electron in their outermost shell while group 2 elements have two electrons in their outermost shell.
Question. Why does atomic size decrease as we move from left to right along a period in the periodic table?
Answer. Effective nuclear charge increases as we move from left to right in the periodic table so atomic size decreases.
Question. Out of Li and K which will have stronger metallic character and why?
Answer. Potassium (K) will have stronger metallic character than lithium (Li) because as we move from top to bottom in a group, the size increases which increases the ease of liberation of electrons.
Question. Write the number of horizontal rows in the Modern Periodic Table. What are these rows called?
Answer. There are seven horizontal rows of elements in the Modern periodic table which are known as periods.
CBSE Class 10 Science Chapter 5 Periodic classification of elements Short Answer Type Questions
Question. The electronic configuration of an element is 2, 8, 4. State its
(a) group and period in the Modern Periodic Table.
(b) name and write its one physical property.
Answer. (a) The element belongs to group 14 and 3rd period of the Modern Periodic Table.
(b) The element is silicon. It is non-lustrous.
Question. Three elements ‘X’, ‘Y’ and ‘Z’ having atomic numbers 11, 7 and 6 respectively react with oxygen to form their oxides.
(a) Arrange these oxides in increasing order of their basic nature.
(b) Give reason for your answer.
Answer. 11X = 2, 8, 1
7Y = 2, 5
6Z = 2, 4
(a) Y < Z < X
(b) X is metallic in nature hence, its oxide is basic in nature.
While Y and Z are non-metals and their oxides are acidic in nature.
Question. Give reason – While going down in group 1 lithium is least electropositive while caesium is most electropositive.
Answer. As we move down in a group, an electron shell is added after every change of period. For example, lithium has only two electron shells around the nucleus, but caesium has six electron shells around the nucleus. Thus, the positive charge within the nucleus holds the valence electrons of lithium more strongly than caesium. In other words, the valence electrons are held very loosely in case of caesium than lithium. Thus, caesium can lose its valence electrons more easily and hence, is more electropositive as compared to lithium, which is less electropositive. In other words, caesium is more metallic in character than lithium.
Question. Calcium is an element with atomic number 20. Stating reason answer each of the following questions :
(i) Is calcium a metal or non-metal?
(ii) Will its atomic radius be larger or smaller than that of potassium with atomic number 19?
(iii) Write the formula of its oxide.
Answer. Given that, atomic number of calcium is 20.
So, its electronic configuration = 2, 8, 8, 2
(i) As, it has 2 valence electrons in the outermost shell which can be easily lost, so it is a metal.
(ii) Atomic number of K (potassium) is 19 so, it is placed before Ca(20) in the same period.
On moving from left to right in a period, the atomic radius decreases.
Hence, atomic radius of Ca(20) will be smaller than that of K(19).
(iii) The valency of calcium as well as oxygen is 2 thus, the formula of the oxide will be CaO.
Question. Chlorine is an element in period 3 of the Periodic Table. Bromine is found in period 4 of the Periodic Table. These two elements may be from different periods of the periodic table, but they have many similar properties.
Element Molecular Number of
formula valence electrons
Chlorine
Bromine
(a) Complete the given table.
(b) Explain why the properties of chlorine and bromine closely resemble one another.
(c) Lithium is an element from Group I of the Periodic Table. Write the formula of the compound formed between lithium and
(i) chlorine
(ii) bromine.
(iii) What type of bonding is found in these compounds? Give reason.
Answer. (a)
Element Molecular formula Number of valence electrons
Chlorine Cl2 7
Bromine Br2 7
(b) Chlorine and bromine are in the same group i.e., Group VII of the Periodic Table.
Chlorine and bromine have seven valence electrons each.Since both have the same number of valence electrons, they have similar chemical properties.
Both readily gain or share one electron to achieve a stable octet configuration.
(c) (i) LiCl (ii) LiBr
(iii) Ionic bonds
Lithium has one valence electron.
Lithium readily loses this valence electron to achieve a noble gas configuration similar to helium.
Li → Li+ + e–
Chlorine and bromine have seven valence electrons each.
Chlorine readily gains one electron to achieve a noble gas configuration similar to argon.
Cl + e– → Cl–
Bromine readily gains one electron to achieve a noble gas configuration similar to krypton.
Br + e– → Br–
Oppositely charged ions are attracted together by strong electrostatic forces of attraction to form ionic bonds.
Question. Potassium, bromine and krypton are elements in period 4 of the Periodic Table.
(a) In which group of the periodic table can these elements be found?
(i) Potassium (ii) Bromine (iii) Krypton
(b) Bromine exists as a molecule. Draw a ‘dotand- cross’ diagram to show the bonding in a molecule of bromine.
(c) Krypton does not react with either potassium or bromine. Explain the unreactive nature of krypton.
Answer. (a) (i) Group I (ii) Group 17 (iii) Group 18
(b) 
(c) Krypton has a stable electronic configuration, with 8 electrons in its valence shell. Hence, it does not lose, gain or share electron(s) with another atom.
Question. How many elements can be accommodated in each period of the periodic table? What are these periods called on the basis of number of elements?
Answer. Based on the maximum capacity of a shell according to the formula 2n2 the number of elements in each period can be given as follows :
n = 1 (Maximum no. of elements 2). Thus, first period has 2 elements. It is called very short period.
n = 2 (Maximum no. of elements 8). Thus, 2nd period has 8 elements. It is called short period.
n = 3 (Maximum no. of elements 8). Thus, 3rd period has 8 elements. It is called short period.
n = 4 (Maximum no. of elements 18). Thus, 4th period has 18 elements. It is called long period.
n = 5 (Maximum no. of elements 18). Thus, 5th period has 18 elements. It is called long period.
n = 6 (Maximum no. of elements 32). Thus, 6th period has 32 elements. It is called very long period.
n = 7 (Maximum no. of elements 32). Thus, 7th period has 32 elements. It is also called very long period.
Question. The atomic number of an element ‘X’ is 20.
(i) Determine the position of the element ‘X’ in the periodic table.
(ii) Write the formula of the compound formed when ‘X’ reacts/combines with another elements ‘Y’ (atomic number 8).
(iii) What would be the nature (acidic or basic) of the compound formed? Justify your answer.
Answer. Atomic number of element X is 20 so, it is calcium (Ca).
Electronic configuration of Ca = 2, 8, 8, 2
(i) As calcium has two valence electrons in its outermost shell, so it belongs to group 2.
Moreover, it has four shells which indicates that it belongs to period number 4.
(ii) Calcium forms a basic oxide having the formula :

(iii) When calcium oxide is treated with water then calcium hydroxide (Basic oxide) is formed.
CaO+H2O → Ca(OH)2
Calcium hydroxide
Question. An element ‘X’ is placed in the 3rd group and 3rd period of the Modern Periodic Table.Answer the following questions stating reason for your answer in each case :
(a) Write the electronic configuration of the element ‘X’.
(b) Write the formula of the compound formed when the element ‘X’ reacts with another element ‘Y’ of atomic number 17.
(c) Will the oxide of this element be acidic or basic ?
Answer. X is placed in 3rd group (IIIA) and 3rd period of the Modern periodic table then it must be aluminium (Al).
As it belongs to 3rd group so it will have 3 electrons in its outermost shell.
Also it belongs to 3rd period, so it will have 3 shells.
(a) Electronic configuration of X = 2, 8, 3
(b) Atomic number of Y is 17
Electronic configuration is 2, 8, 7
Valency of Y = 8 – 7 = 1
Formula of compound formed when X reacts with Y is

(c) Al2O3 is amphoteric in nature i.e., acidic as well as basic oxide.
Question. (a) Which is more basic
(i) K2O or Na2O
(ii) K2O or CaO?
(b) Name a species that will be isoelectronic with each of the following atoms or ions :
(i) Ne (ii) Cl–
(iii) Ca2+ (iv) Rb
Answer. (a) (i) K2O
Potassium (K) and sodium (Na) belong to same group and the basic nature of oxides increases down the group. Therefore, K2O is more basic than Na2O.
(ii) K2O Potassium (K) and calcium (Ca) belong to the same period and basic nature of oxides decreases from left to right in a given period. Therefore, K2O is more basic than CaO
(b) (i) Na+ (ii) S2–
(iii) K+ (iv) Sr2+
Question. Na, Mg and Al are the elements of the 3rd periods of the Modern Periodic Table having group number 1, 2 and 13 respectively. Which one of these elements has the (a) highest valency,
(b) largest atomic radius, and (c) maximum chemical reactivity? Justify your answer stating the reason for each.
Answer. Period number of Na, Mg and Al is 3
Group number of Na, Mg and Al are 1, 2 and 13 respectively.
(a) Aluminium (Al) will show highest valency of 3 as it belongs to group number 13 (valency = 13 – 10 = 3).
Moreover, along the period from left to right valency first increases to maximum and then decreases.
(b) Sodium (Na) will have the largest atomic radius because as we move along the period from left to right, the atomic radius decreases.
(c) Sodium (Na) will have maximum chemical reactivity because as we move along the period from left to right, chemical reactivity decreases.
Question. The second period of the long form of periodic table contains the following elements :
Li B e B C N O F N e
(a) Write down their electronic configurations.
(b) Do they contain the same number of valence electrons?
(c) Do they contain the same number of shells?
Answer. (a) Electronic configurations of the elements :
Li Be B C N O F Ne
2,1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8
(b) These elements do not contain same number of valence electrons.
(c) They contain same number of shells (K and L).
Question. In the following table six elements A, B,C, D, E and F (here letters are not the usual symbols of the elements) of the Modern Periodic
Table with atomic number 3 to 18 are given :
(a) Which of these
(i) a noble gas, (ii) a halogen?
(b) If B combines with F, what would be the formula of the compound formed?
(c) Write the electronic configurations of C and E.
Answer. (a) (i) Noble gas = G
(ii) Halogen = F
(b) B(11) = 2, 8, 1
F(17) = 2, 8, 7
Valency of B = 1
Valency of F = 8 – 7 = 1
Formula of the compound formed :
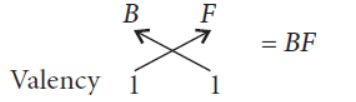
(c) Electronic configuration of C(12) = 2, 8, 2
Electronic configuration of E(8) = 2, 6
Question. The atomic number of an element is 16. Predict
(i) the number of valence electrons in its atom
(ii) its valency
(iii) its group number
(iv) whether it is a metal or a non-metal
(v) the nature of oxide formed by it
(vi) the formula of its chloride.
Answer. Atomic number of element (E) is 16
Electronic configuration = 2, 8, 6
(i) Number of valence electrons in its atom = 6
(ii) Valency = 8 – 6 = 2
(iii) As there are 6 valence electrons thus, its group number is 10 + 6 = 16
(iv) This element is a non-metal.
(v) The nature of oxide formed by this element is acidic.
(vi) The formula of the chloride of non-metal ‘E’ will be

Question. The positions of three elements A, B and C in the periodic table are indicated below :
Group 16 Group 17
– – (First period)
– A (Second period)
– – (Third period)
B C (Fourth period)
(a) State whether element C would be a metal or a non-metal? Why?
(b) Which is the more active element A or C? Why?
(c) Which type of ion (cation or anion) will be formed by the element C? Why?
Answer. (a) C belongs to group 17 and hence, it will have 7 valence electrons in the outermost shell and has a tendency to gain electrons thus, it is a non-metal.
b) Among A and C, A will be more reactive as the reactivity decreases down the group. So, A has more tendency to gain electrons.
(c) C will form negatively charged ion which is known as anion because group 17 elements have seven electrons in their outermost shell so, they have strong tendency to gain an electron to attain the noble gas configuration.
Question. Element Y is given the chemical symbol 4020Y.
(a) What is the electronic configuration of element Y?
(b) Determine the position of element Y in the periodic table.
(c) Explain how an atom of element Y can form an ion.
Answer. (a) Proton number = 20 = number of electrons Electronic configuration = 2, 8, 8, 2
(b) Element Y is in group II and period 4 because it has 2 electrons in outermost shell and four occupied shells.
(c) It is easier for an atom of element Y to lose the two valence electrons to achieve an electronic configuration similar to argon (2, 8, 8). Hence, an atom of element Y will form a positive ion with charge equal to its group number, i.e. 2. The formula of the ion is Y2+.
Question. (a) How does metallic character of elements in Modern Periodic Table vary on moving from
(i) left to right in a period
(ii) top to bottom in a group?
Explain with the help of an example in each case.
(b) If an element X is placed in group-14, what will be the nature of bond in its chloride? Write the chemical formula of the compound formed.
(c) An element X has mass number = 35 and number of neutrons = 18. What is the atomic number of X? Write electronic configuration of X and determine its valency.
Answer. (a) In the Modern periodic table, there are 18 vertical columns called groups and 7 horizontal rows called periods.
Trend of metallic character :
(i) Along the period from left to right : Metallic character of elements decreases as we move from left to right in a period.
Metallic character depends on the electropositive character (tendency to lose electrons) of the elements. As we go across the period from left to right, one electron is added to same shell at every stage which increases the effective nuclear charge and hence, valence electrons becomes more and more closer to the nucleus. Due to this, the tendency of atoms to lose valence electrons and form positive ions decreases. Hence, electropositive character decreases resulting in decrease of metallic character.
Example :
Variation of metallic character across the period :

(ii) Down the group : Metallic character of elements increases on moving down the group as the electropositive character increases down the group.
Example :
Variation of metallic character down the group :

(b) Since element ‘X’ is placed in group 14, therefore, its valency is 14 – 10 = 4. Further, since it is difficult to either lose all the four valence electrons or gain four more electrons, therefore, it prefers to share these four electrons to acquire the stable electronic configuration of the nearest inert gas.
Thus, the nature of the bond of chloride of element ‘X’ is covalent and the chemical formula is XCl4.
(c) Mass number of X = 35
Number of neutrons = 18
Number of electrons = Number of protons
= (Mass number – Number of neutrons)
= 35 – 18 = 17
Number of electrons of X = Atomic number of X = 17
Thus, electronic configuration of X = 2, 8, 7
As it has 7 electrons in the outermost shell, so it belongs to 17th group. Moreover the electrons are present in three shells, so it belongs to 3rd period. Valency of X = 8 – 7 = 1
Question. The table below shows the electronic configuration of six elements.
| Element | Electronic configuration |
| P | 2,1 |
| Q | 2,4 |
| R | 2,7 |
| S | 2,8,7 |
| T | 2,8,8 |
| U | 2,8,8,1 |
(a) Give the letter (s) of
(i) two elements that are in the same period of the periodic table.
(ii) two elements that are in the same group of the periodic table.
(iii) a noble gas.
(iv) group VII non-metals.
(v) element which forms positive ion.
(b) (i) Give the formula of the compound formed between elements P and S.
(ii) What type of bonding would you expect in the compound formed?
Answer. (a) (i) P, Q and R (two electron shells) or S and T (three electron shells).
(ii) P and U (one valence electron) or R and S (seven valence electrons).
(iii) T (completely filled valence shell).
(iv) R or S (seven valence electrons).
(v) P or U (one valence electron).
(b) (i) PS
(ii) ionic bonding.
Question. (a) The modern periodic table has been evolved through the early attempts of Dobereiner, Newlands and Mendeleev. List one advantage and one limitation of all the three attempts.
(b) Name the scientist who first of all showed that atomic number of an element is a more fundamental property than its atomic mass.
(c) State Modern periodic law.
Answer. (a) Advantage of Dobereiner’s triads : It recognised a
relationship between properties of elements and their atomic weights.
Limitation of Dobereiner’s triads : Dobereiner could identify only three triads. He was not able to prepare triads of all the known elements.
Advantages of Newland’s law of octaves : This law provided a basis for the classification of elements into groups of elements having similar properties.
Limitation of Newlands’ law of octaves : This law worked only for the lighter elements. All the element discovered at that time could not be classified into octaves.
Advantages of Mendeleev’s periodic table : He classified all the 63 elements discovered at that time on the basis of similarities in their properties.
Limitation of Mendeleev’s periodic table : Increasing order of atomic masses could not be maintained in all cases e.g., cobalt with higher atomic mass was placed before nickel.
(b) Henry Moseley, an English physicist, showed that atomic number of an element is a more fundamental property than its atomic mass.
(c) Modern periodic law states that the physical and chemical properties of elements are the periodic function of their atomic numbers.
Please click the link below to download CBSE Class 10 Science Periodic classification of elements Notes.
| CBSE Class 10 Science Electricity |
| CBSE Class 10 Science Electricity Notes |
| CBSE Class 10 Science Electricity Sure Shot Questions A |
| Class 10 Science Electricity Exam Notes |
CBSE Class 10 Science Chapter 5 Periodic Classification of Elements Study Material
We hope students liked the above Study Material for Chapter 5 Periodic Classification of Elements designed as per the latest syllabus for Class 10 Science released by CBSE. Students of Class 10 should download the Study Material in Pdf format, read the notes and related questions and solutions given in above Class 10 Science Study Material on daily basis. All latest Study Material have been developed for Science by referring to the most important and regularly asked topics which the students should learn and practice to get better score in school tests and examinations. Expert teachers of studiestoday have referred to NCERT book for Class 10 Science to develop the Science Class 10 Study Material. After solving the questions given in the Study Material which have been developed as per latest course books also refer to the NCERT solutions for Class 10 Science designed by our teachers. Also download Class 10 Science Sample Papers given on studiestoday. After solving these you should also refer to Class 10 Science MCQ Test for the same chapter.
You can download free study material for Class 10 Science Chapter 5 Periodic Classification of Elements for latest academic session from StudiesToday.com
Yes, the study material given here for Class 10 Science Chapter 5 Periodic Classification of Elements is for current CBSE session
All study maetrial for CBSE Class 10 Science Chapter 5 Periodic Classification of Elements is free

