Read and download free pdf of CBSE Class 10 Chemistry Carbon And Its Compounds Worksheet Set E. Download printable Science Class 10 Worksheets in pdf format, CBSE Class 10 Science Chapter 04 Carbon and Its Compounds Worksheet has been prepared as per the latest syllabus and exam pattern issued by CBSE, NCERT and KVS. Also download free pdf Science Class 10 Assignments and practice them daily to get better marks in tests and exams for Class 10. Free chapter wise worksheets with answers have been designed by Class 10 teachers as per latest examination pattern
Chapter 04 Carbon and Its Compounds Science Worksheet for Class 10
Class 10 Science students should refer to the following printable worksheet in Pdf in Class 10. This test paper with questions and solutions for Class 10 Science will be very useful for tests and exams and help you to score better marks
Class 10 Science Chapter 04 Carbon and Its Compounds Worksheet Pdf
CARBON: INTRODUCTION
Carbon is the fourth most abundant element in the universe by mass. It is also the second most abundant element in the human body after oxygen. It is the 15th most common element in the Earth’s crust. Carbon was discovered in prehistory and it was known to the ancients. They used to manufacture charcoal by burning organic material.
Carbon is a non-metal. It belongs to the fourteenth group or IV A group in the modern periodical table. The elements of this group have four electrons in the valence shell.
Atomic Number: 6
Electronic configuration: 2, 4
Valence electrons: 4
Property: Non-metal
Compounds having carbon atoms among the components are known as carbon compounds.
Previously, carbon compounds could only be obtained from a living source; hence they are also known as organic compounds.
BONDING IN CARBON: COVALENT BOND
Bond formed by sharing of electrons is called covalent bond. Two of more atoms share electrons to make their configuration stable. In this type of bond, all the atoms have similar
rights over shared electrons. Compounds which are formed because of covalent bond are called COVALENT COMPOUNDS.
FORMATION OF COVALENT BONDS
1. Covalent band is the chemical bond formed through the sharing of electrons between two non-metal atoms.
2. Compounds which have covalent bonds are called covalent compounds.
3. Examples of covalent compounds or molecules are chlorine, Cl2, carbon dioxide, CO2, ammonia, NH3, water, H2O, and tetrachloromethane (carbon tetrachloride), CCl4.
4. During the formation of covalent molecules, each non-metal covalent atom provides one, two or three electrons to be shared with other atoms. The bond formed is called a covalent bond.
5. Through this process, each non-metal atom in covalent molecules will achieve stable electron arrangement.
6. The type of covalent bond formed in a covalent compound depends on the number of electron pairs shared between non-metal atoms.
Covalent bonds are of three types: Single, double and triple covalent bond.
SINGLE COVALENT BOND
1. A single covalent bond is the covalent bond formed through the sharing of a pair of electrons between two non-metal atoms.
2. Each non-metal atom contributes one electron for sharing to achieve a stable electron arrangement.
3. Example of single covalent compound are chlorine gas, Cl2, hydrogen chloride, HCl, water, H2O, methane, CH4, ammonia, NH3, and tetrachloromethane, CCl4.
4. Single covalent bonds can also be formed between different non-metal atoms.
Formation of hydrogen molecule (H2)
Atomic Number of H = 1
Electronic configuration of H = 1
Valence electron of H = 1
Hydrogen forms a duet, to obtain stable configuration. This configuration is similar to helium (a noble gas).
Since, hydrogen has one electron in its valence shell, so it requires one more electron to form a duet. So, in the formation of hydrogen molecule; one electron from each of the hydrogen atoms is shared.
Formation of hydrogen chloride (HCl):
Valence electron of hydrogen = 1
Atomic number of chlorine = 17
Electronic configuration of chlorine: 2, 8, 7
Electrons in outermost orbit = 7
Valence electron = 7
Formation of water (H2O)
Valence electron of hydrogen = 1
Atomic number of oxygen = 8
Electronic configuration of oxygen = 2, 6
Valence electron = 6
DOUBLE COVALENT BOND
1. Double covalent bond is the type of covalent bond formed through the sharing of two pairs of electrons between two non-metal atoms.
2. Examples of molecules which have double covalent bonds are oxygen, O2, and carbon dioxide, CO2.
3. During the formation of double bond, each non-metal atom contributes two pairs of electrons to be shared to achieve a stable electron arrangement.
Formation of oxygen molecule (O2):
Valence electron of oxygen = 2
♦ In the formation of oxygen molecule, two electrons are shared by each of the two oxygen atoms to complete their stable configuration.
♦ In oxygen, the total number of shared electrons is four, two from each of the oxygen atoms.
So a double covalent bond is formed.
Formation of Carbon dioxide (CO2):
Valence electron of carbon = 4
Valence electron of oxygen = 6
TRIPLE COVALENT BOND
1. The triple covalent bond is the type of covalent bond formed through the sharing of three pairs of electrons between two non-metal atoms.
2. Example of molecule which has triple covalent bonds is the nitrogen molecule, N2.
Formation of Nitrogen (N2):
Atomic number of nitrogen = 7
Electronic configuration of nitrogen = 2, 5
Valence electron = 5
In the formation of nitrogen, three electrons are shared by each of the nitrogen atoms. Thus one triple bond is formed because of the sharing of total six electrons.
Properties of Covalent Bond:
♦ Intermolecular force is smaller.
♦ Covalent bonds are weaker than ionic bond. As a result, covalent compounds have low melting and boiling points.
♦ Covalent compounds are poor conductor of electricity as no charged particles are formed in covalent bond.
♦ Since, carbon compounds are formed by the formation of covalent bond, so carbon compounds generally have low melting and boiling points and are poor conductor of electricity.
ALLOTROPY
Allotropy is defined as the property by which an element can exist in more than one form that are physically different but chemically similar.
Allotropes of carbon
Carbon exists in three allotropic forms. They are crystalline form (diamond and graphite), amorphous form (coke,charcoal) and fullerene.
In diamond each carbon atom is bonded to four other carbon atoms forming a rigid three dimensional structure , accounting for it’s hardness and rigidity.
General properties of diamond are
♦ It is a colourless transparent substance with extraordinary brilliance due to its high refractive index.
♦ It is quite heavy.
♦ It is extremely hard (hardest natural substance known).
♦ It does not conduct electricity (because of the absence of free electrons).
♦ It has high thermal conductivity and high melting point.
♦ It burns on strong heating to form carbon dioxide.
In graphite each carbon atom is bonded to three other carbon atoms in the same plane giving hexagonal layers held together by weak vander Waals forces accounting for softness.
General properties of graphite are
♦ It is a greyish black opaque substance.
♦ It is lighter than diamond, feels soft and slippery to touch.
♦ It is a good conductor of electricity (due to the presence of free electrons) but bad conductor of heat.
♦ It burns on strong heating to form carbon dioxide.
Fullerenes form another type of carbon allotropes. The first one was identified to contain 60 carbon atoms in the shape of a football. (C-60). Since this looks like the geodesic dome designed by the US architect Buck Minster Fuller, it is named as Buck Minster Fullerene.
General Properties of fullerenes are
♦ These are dark solids at room temperature.
♦ These are neither too hard nor too soft.
♦ These are the purest allotrophic forms of carbon because of the absence of free valencies or surface bonds.
♦ On burning, these produce only carbon dioxide gas.
VERSATILE NATURE OF CARBON
Initially, compounds of carbon could only be obtained from living sources and there was no way of synthesizing them. Hence, carbon compounds are also known as organic compounds. Carbon forms a large number of compounds. So far, formulae of about 3 million carbon compounds are known.
Cause of formation of such a large number of compounds by carbon:
♦ Carbon can form bonds with other carbon atoms. This property of carbon is known as CATENATION. Because of catenation, carbon can form a long chain; while making bond with other carbon atoms. Carbon can make single, double and triple bonds by catenation.
♦ Carbon can form branched chain; along with straight chain; while combining with carbon atoms, i.e. because of the property of catenation.
♦ Due to the valency of four, carbon is capable of bonding or pairing with four other carbon atoms or with the atoms of some other monovalent elements. It also forms compounds with oxygen, nitrogen, sulphur, hydrogen and many other elements. This gives rise to compounds with specific properties which depend on the element other than carbon present in the molecule.
♦ Bonds which carbon forms with other elements are very strong thus, making these compounds very stable. The main reason for such strong bond formation is the small size of carbon. As a result, the shared pair of electrons are tightly held by the nucleus.
ORGANIC COMPOUNDS
The compounds of carbon except its oxides, carbonates and hydrogen carbonate salts, are known as organic compounds. These compounds were initially extracted from natural
substances and was believed that some vital force was necessary for the synthesis of these compounds (vital force theory).
HYDROCARBONS
(Hydrogen + Carbon = Hydrocarbon) Compounds formed because of the combination of hydrogen and carbon are known as hydrocarbons. These are regarded as the parent organic compounds and all other compounds are considered to be derived from them by the replacement of one or more hydrogen atoms by other atoms or groups of atoms.
Hydrocarbons can be divided into various classes as shown in below:
ALIPHATIC HYDROCARBONS
The word aliphatic is derived from the Greek word aleiphar meaning fat. Aliphatic hydrocarbons were named so because they were derived from fats and oils. Hydrocarbons can be acyclic compounds, which are straight chain compounds, or cyclic compounds, which have rings of carbon atoms.
AROMATIC HYDROCARBONS
The word aromatic is derived from the word aroma meaning fragrance. The aromatic compounds have a characteristic smell. Structurally, they include benzene and its derivative.
The aliphatic hydrocarbons can be divided into two categories: saturated hydrocarbons and unsaturated hydrocarbons. In saturated hydrocarbons, carbon atoms are linked to each other by single bonds whereas in unsaturated hydrocarbons, multiple bond (double and triple bonds) are present between carbon atoms.
SATURATED HYDROCARBONS
Alkanes
General formula = CnH2n+2 Suffix : ane
These are the organic compounds which contain carbon – carbon single bond. These were earlier named as paraffins(Latin : meaning little affinity) due to their least chemical reactivity. According to IUPAC system, these are named as alkanes (ane is suffix with root word).
UNSATURATED HYDROCARBONS
These are hydrocarbons which contain carbon to carbon double bonds or carbon to carbon triple bonds in their molecules.These are further classified into two types: alkenes and alkynes.
i)Alkenes: General formula: CnH2n Suffix : ene
The hydrocarbons containing atleast one carbon to carbon double bond are called alkenes.They have the general formula CnH2n .These were previously called olefins (Greek : olefiant – oil forming) because the lower gaseous members of the family form oily products when treated with chlorine.
In IUPAC system, the name of alkene is derived by replacing suffix “ane” of the correspding alkane by “ene”. For example,
CH3 - CH3 H2C = CH2
ethane ethene
In higher alkenes, the position of the double bond, can be indicated by assigning numbers 1, 2, 3, 4, ……to the carbon atoms present in the molecule.
ii) Alkynes: General formula: CnH2n-2 Suffix : yne
The hydrocarbons containing carbon to carbon triple bond are called alkynes. Alkynes are named in the same way as alkenes i.e., by replacing suffix ane of alkane by yne. In higher
members, the position of triple bond is indicated by giving numbers 1, 2, 3, 4, ….to the carbon atom in the molecule.
HOMOLOGOUS SERIES
A homologous series is a group or a class of organic compounds having similar structure and similar chemical properties in which the successive compounds differ by a CH2 group.
Characteristics of homologous series
♦ Each member of the series differs from the preceeding or succeeding member by a common difference of CH2 and by a molecular mass of 14 amu (amu = atomic mass unit).
♦ All members of homologous series contain same elements and the same functional groups.
♦ All members of homologous series have same general molecular formula.
e.g Alkane = CnH2n + 2
Alkene = CnH2n
Alkyne = CnH2n – 2
♦ The members in homologous series show a regular gradation in their physical properties with respect to increase in molecular mass.
♦ The chemical properties of the members of the homologous series are similar.
♦ All members of homologous series can be prepared by using same general method.
IMPORTANCE OF HOMOLOGOUS SE RIES
♦ It helps to predict the properties of the members of the series that are yet to be prepared.
♦ Knowledge of homologous series gives a systematic study of the members.
♦ The nature of any member of the family can be ascertained if the properties of the first member are known.
FUNCTIONAL GROUP
Functional group may be defined as an atom or group of atoms or reactive part which is responsible for the characteristic properties of the compounds.
The chemical properties of organic compounds are determined by the functional groups while their physical properties are determined by the remaining part of the molecule.
CLASSIFICATION OF ORGANIC COMPOUNDS BASED ON FUNCTIONAL GROUP
1. ALCOHOLS
Alcohols are carbon compounds containing –OH group attached to alkyl group. The general formula of alcohol is R-OH where ‘R’ is an alkyl group and –OH is the functional group.
The IUPAC name of alcohol is derived by replacing –e, in the word alkane, by the suffix –ol.
Hence we get the name alkanol.
2. ALDEHYDES
Aldehydes are carbon compounds containing -CHO group attached to alkyl group or hydrogen atom. The general formula of aldehydes is R – CHO where ‘R’ is an alkyl group or hydrogen atom and – CHO is the functional group.
The IUPAC name of aldehyde is derived by replacing –e, in the word alkane, by the suffix –al.
Hence we get the name “alkanal”.
3. KETONES
Ketones are carbon compounds containing carbonyl – CO – group attached to two alkyl groups. The general formula of ketone is R-CO-R’ where R and R’ are alkyl groups and – CO – is the functional group.
The IUPAC name of ketone is derived by replacing –e, in the word alkane, by the suffix -one.
Hence we get the name “alkanone”.
4. CARBOXYLIC ACIDS
Carboxylic acids are carbon compounds containing –COOH group attached to a hydrogen atom or alkyl group. The general formula of acid is R-COOH where ‘R’ is a hydrogen atom or alkyl group and –COOH is the functional group.
The IUPAC name of acid is derived by replacing – e, in the word alkane, by the suffix –oic acid. Hence we get the name “alkanoic acid”.
ISOMERISM
Carbon compounds or organic compounds with same molecular formula can show different structures and hence, different properties. This phenomenon is called isomerism and compounds are called isomers.
For example, following two arrangements are possible for butane, an alkane with four C atoms (C4H10)
Such pair of isomers is called chain isomers and the isomerism is called chain isomerism.
Thus, chain isomers are the compounds that have same molecular formula but differ in the arrangement of carbon chains.
NOMENCLATURE OF CARBON COMPOUNDS
In general, the names of organic compounds are based on the name of basic carbon chain modified by a prefix (phrase before) or suffix (phrase after) showing the name of the functional group.
Following steps are used to write the name of an organic compound
Step 1 Count the number of carbon atoms in the given compound and write the root word for it (Root word upto 10 carbon atoms are tabulated below.)
Step 2 If the compound is saturated, add suffix ‘ane’ to the root word, but if is unsaturated, add suffix ‘ene’ and ‘yne’ for double and triple bonds respectively.
For example,CH3CH2CH3 contains three C atoms so root word is ‘prop’ and it contains only single bonds, so suffix used is ‘ane’. Hence, the name of this compound is propane.
Similarly, the compound CH3CH == CH2 is named as propene as here suffix ‘ene’ is used for double bond.
Step 3 If functional group is present in the compound, it is indicated by adding its suffix (which are given in the table above).
♦ Prefix ‘iso’ and ‘neo’represent the presence of one or two carbon atoms respectively as side chain.
♦ If the functional group is named as a suffix, the final ‘e’ of alkane (or alkene or alkyne) is substituted by appropriate suffix.
♦ If the functional group and subtituents are not present at first carbon, then their location is indicated by digits 1,2,3... .
INTEXT QUESTIONS PAGE NO. 68
Q1. How many structural isomers can you draw for pentane?
Ans: Pentane (C5H12) has a skeleton of five carbon atoms. It can exist as straight chain as well as two branched chains. The possible structural isomers have been shown below.
Q2. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Ans: The two features of carbon that give rise to a large number of compounds are as follows:
(i) Catenation − It is the ability to form bonds with other atoms of carbon.
(ii) Tetravalency − With the valency of four, carbon is capable of bonding with four other atoms.
Q3. What will be the formula and electron dot structure of cyclopentane?
Ans: General formula of cycloalkane = CnH2n
In cyclopentane n = 5,
∴ Formula of cyclopentane, C5H5 × 2 = C5H10
Electron dot structure of cyclopentane
Q4. Draw the structures for the following compounds.
(i) Ethanoic acid (ii) Bromopentane*
(iii) Butanone (iv) Hexanal.
Are structural isomers possible for bromopentane?
Ans:
(i) Ethanoic acid
Q5. How would you name the following compounds?
Ans:
(a) CH3 – CH2 – Br
Bromoethane (because for two carbons, root word is ‘eth’)
Formaldehyde or methanal (because for single carbon, root word is ‘meth’)
(c) CH3CH2CH2CH2CHC≡≡C,
1-hexyne (because for 6 carbons, root word is ‘hex’ and for triple bond suffix is ‘yne’.)
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
COMBUSTION
All the carbon compounds burn in oxygen and yield carbon dioxide and water vapour. Heat and light are also released during this process. This reaction is called combustion.
(i) C + O2 → CO2 + heat and light
(ii) CH4 + O2 → CO2 + H2O + heat and light
(iii) CH3CH2OH + O2 → CO2 + H2O + heat and light
Further, once carbon and its compounds ignite, they keep on burning without the requirement of additional energy. That’s why these compounds are used as fuels.
Saturated hydrocarbons give a clean flame due to their complete combustion whereas, unsaturated hydrocarbons give a yellow flame with lots of black smoke as they do not undergo complete combustion.
OXIDATION
Oxidation is a process of intake of oxygen and removal of hydrogen. Those substances which are capable of providing oxygen to other substances are called oxidising agents. e.g., alk. KMnO4 and acidified K2Cr2O7 can both behave as oxidising agents.
ADDITION REACTION
The reaction in which a reagent adds completely on a substance without the removal of small molecules are called addition reactions. For example, addition of hydrogen (in the presence of catalysts like Palladium or Nickel) to unsaturated hydrocarbons, yields saturated hydrocarbons (Hydrogenation).
Hydrogenation (addition of hydrogen) of vegetable oil (which are unsaturated compounds) in the presence of nickel catalyst gives ghee (saturated compounds). This process is called hardening of oils.
SUBSTITUTION REACTION
The reactions in which a reagent substitutes (replaces) an atom or a group of atoms from the reactant (substrate) are called substitution reactions. These are generally shown by saturated compounds and benzene.
Most of the saturated hydrocarbons are fairly inert and unreactive in the presence of most reagents. So, presence of sunlight is necessary for their substitution reactions.
When chlorine is added to hydrocarbons at a rapid rate, in the presence of sunlight, Cl replaces H atom one by one.
FUELS AND FLAMES
FUELS
Those carbon compounds which have stored energy in them and burn with heat and light are called fuels. The released energy (heat or light) is utilised for various purposes like for cooking food, running machines in factories, etc. In fuels, the carbon can be in free state as present in coal, coke and charcoal or in combined state as present in petrol, LPG, kerosene, petroleum, natural gas, etc. Those fuels which were formed by the decomposition of the remains of the pre-historic plants and animals (fossils) burried under the earth long ago, are called fossils fuels.
For example, coal, petroleum and natural gas.
COAL
It is a complex mixture of compounds of carbon, hydrogen and oxygen and some free carbon alongwith traces of nitrogen and sulphur.
It was formed by the decomposition of plants and trees buried under the earth millions of years ago.
PETROLEUM
It is a dark viscous foul smelling oil and is also known as rock oil or black gold. It was formed by the decomposition of the remains of extremely small plants and animals buried under the sea millions of years ago.
FLAME
A flame is the region where combustion (or burning) of gaseous substances takes place.
Depending upon the amount of oxygen available and burning of fuels, flames are of following two types
(i) Blue or Non-luminous Flame
When the oxygen supply is sufficient, the fuels burn completely producing a blue flame. Since, light is not produced during this type of combustion, so the flame is called non-luminous (nonlight giving flame), e.g., burning of LPG in gas stove.
(ii) Yellow or Luminous Flame
In the insufficient supply of air, the fuels burn incompletely and produce yellow flame. The colour of the flame is yellow because of the presence of unburnt carbon particles. This flame produces light so also known as luminous flame. e.g., burning of wax vapours.
MULTIPLE CHOICE QUESTIONS
Question. Name the metal that is not present in carbon family
a) Si
b) Ge
c) Sb
d) Sn
Answer : C
Question. Which among the following is an unsaturated molecule that has the molecular formula of a cycloalkane.
a) C3H6
b) C8H18
c) C5H12
d) C3H4
Answer : A
Question. which of the following statements is correct about the given electron dot structure
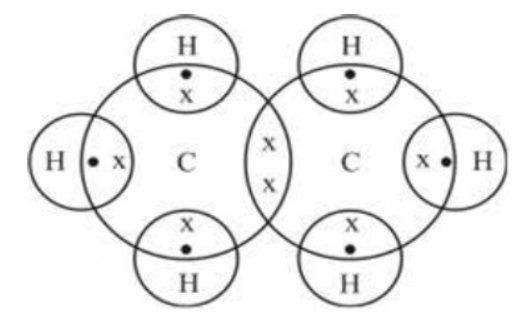
a. The compound has 8 bonds of which one is a double bond
b. The compound is formed of all single bonds of which one is a C-C bond.
c. The electrons in every shell of the atoms are shown in the structure.
d. Electron dot structure doesn't help to identify the bonds in a compound
Answer : B
Question. Which of the following is unsaturated molecule?
a) C3H8
b) C2H2
c) C5H12
d) C4H10
Answer : B
Question. 3rd homologue of alkyne series is---
a) Propyne
b) propene
c) butyne
d) butane
Answer : C
Question. Number of covalent bonds in cyclobutane is
a) 12
b) 10
c) 4
d)14
Answer : A
Question. Which of the following is not an alkane, alkene or alkyne?
a) CH4
b) C2H2
c) CH3
d) C5H8
Answer : C
Question. Which of the statement regarding homologous series is wrong?
a) only alkanes have homologous series
b) consecutive members of a homologous series differ by an atomic mass of 14u
c) A given homologous series can be expressed by a general formula
d) Homologues of a given series show gradation in physical properties.
Answer : A
Question. Which of the following homologue does not belong to a given homologous series
a) C5H12
b) C8H18
c) CH4
d) C2H4
Answer : D
CASE STUDY BASED
Read the following and answer any four questions
The compounds which have the same molecular formula but differ from each other in physical or chemical properties are called isomers and the phenomenon is called isomerism. When the isomerism is due to difference in the arrangement of atoms within the molecule, without any reference to space, the phenomenon is called structural isomerism. In other words. Structural isomers are compounds that have the same molecular formula but different structural formulas, i.e., they are different in the order in which different atoms are linked. In these compounds, carbon atoms can be linked together in the form of straight chains, branched chains or even rings.
Question. Which of the following sets of compounds have same molecular formula?
(a) Butane and iso-butane
(b) Cyclohexane and hexene
(c) Propanal and propanone
(d) All of these
Answer : D
Question. In order to form branching, an organic compound must have a minimum of
(a) four carbon atoms
(b) three carbon atoms
(c) five carbon atoms
(d) any number of carbon atoms.
Answer : A
Question. Which of the following is an isomeric pair?
(a) Ethane and propane
(b) Ethane and ethene
(c) Propane and butane
(d) Butane and 2-methylpropane
Answer : D
Question. Among the following the one having longest chain is
(a) neo-pentane
(b) iso-pentane
(c) 2-methylpentane
(d) 2,2-dimethylbutane.
Answer : C
Question. The number of isomers of pentane is
(a) 2
(b) 3
(c) 4
(d) 5
Answer : B
Read the following and answer any four questions
A series of organic compounds having the same functional group, with similar or almost identical chemical characteristics in which all the members can be represented by the same general formula and the two consecutive members of the series differ by -CH2 group or 14 mass unit in their molecular formulae is called a homologous series. For example, all the members of the alcohol family can be represented by the general formula, CnH2n+1OH where n may have the values 1, 2, 3, etc. The various members of a particular homologous series are called homologues. The physical properties such as density, melting point, boiling point, solubility etc. of the members of a homologous series show almost regular variation in ascending and descending the series.
Question. Which one of the following is not a characteristic of members of a homologous series?
(a) They possess varying chemical properties.
(b) Their physical properties vary in a regular and predictable manner.
(c) Their formulae fit the general molecular formula
(d) Adjacent members differ by one carbon and two hydrogen atoms
Answer : A
Question. All the members of homologous series of alkynes have the general formula
(a) CnH2n
(b) CnH2n+2
(c) CnH2n-2
(d) CnH2n-4
Answer : C
Question. Which of the following statements is not correct?
(a) A common functional group is present in different members of a homologous series.
(b) Two consecutive members of a homologous series differ by a -CH3 group.
(c) The molecular mass of a compound in the series differs by 14 a.m.u. from that of its neighbour.
(d) All the members of a homologous series have common general methods of preparation.
Answer : B
Question. Identify the correct statements:
(a) As the molecular mass increases in any homologous series, a gradation in physical properties is seen.
(b) The melting and boiling points decrease with increasing molecular mass.
(c) As the molecular mass increases in any homologous series, variation in chemical properties is observed a gradation in physical properties is seen.
(d) Adjacent members in a homologous series differ by 18u.
Answer : A
INTEXT QUESTIONS PAGE NO. 71
Q1. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Ans: Since the conversion of ethanol to ethanoic acid involves the addition of oxygen to ethanol, it is an oxidation reaction.
Q2. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Ans: When ethyne is burnt in air, it gives a sooty flame. This is due to incomplete combustion caused by limited supply of air. However, if ethyne is burnt with oxygen, it gives a clean flame with temperature 3000°C because of complete combustion. This oxy-acetylene flame is used for welding. It is not possible to attain such a high temperature without mixing oxygen. This is the reason why a mixture of ethyne and air is not used.
SOME IMPORTANT CARBON COMPOUNDS – ETHANOL AND ETHANOIC ACID
Almost all the compounds are useful to us in a number of ways. Most of the fuels, medicines, paints, explosives, synthetic polymers, perfumes and detergents are basically organic compounds. In fact, organic chemistry has made our life colourful and also comfortable.
Two commercially important compounds are ethanol and ethanoic acid
ETHANOL (C2H5OH)
Ethanol or ethyl alcohol or simply alcohol is one of the most important members of the family of alcohols.
(1) Manufacture of ethanol from molasses
Molasses is a dark coloured syrupy liquid left after the crystallization of sugar from the concentrated sugar cane juice. Molasses still contain about 30% of sucrose which can not be separated by crystallization. It is converted into ethanol by the following steps:
(i) Dilution
Molasses is first diluted with water to bring down the concentration of sugar to about 8 to 10 percent.
(ii) Addition of ammonium salts
Molasses usually contains enough nitrogenous matter to act as food for yeast during fermentation. If the nitrogen content of the molasses is poor, it may be fortified by the addition
of ammonium sulphate or ammonium phosphate.
(iii) Addition of yeast
The solution from step (ii) is collected in large ‘ fermentation tanks’ and yeast is added to it.
The mixture is kept at about 303K for a few days.During this period, the enzymes invertase and zymase present in yeast, bring about the conversion of sucrose into ethanol.
The fermented liquid is technically called wash.
♦ FERMENTATION is the slow chemical change taking place in an organic compound by the action of enzymes leading to the formation of smaller molecules.
(iv) Distillation of wash
The fermented liquid containing 15 to 18 percent alcohol and the rest of the water, is now subjected to fractional distillation. The main fraction drawn, is an aqueous solution of ethanol which contains 95.5% of ethanol and 4.5% of water. This is called rectified spirit. This mixture is then heated under reflux over quicklime for about 5 to 6 hours and then allowed to stand for 12 hours. On distillation of this mixture, pure alcohol (100%) is obtained. This is called absolute alcohol.
PROPERTIES OF ETHANOL
PHYSICAL PROPERTIES
(i) Ethanol is a clear liquid with burning taste.
(ii) Its boiling point is 351K which is higher than corresponding alkane.
(iii) It is completely miscible with water in all proportions.
CHEMICAL PROPERTIES
(i) DEHYDRATION
(a) Intra molecular dehydration : Ethanol, when heated with excess conc. H2SO4 at 443 K undergoes intra molecular dehydration (i.e. removal of water within a molecule of ethanol).
During this reaction, orange colour of K2Cr2O7 changes to green. Therefore, this reaction can be used for the identification of alcohols.
(iv) Esterificaiton : Ethanol reacts with ethanoic acid in the presence of conc.H2SO4 (catalyst) to form ethyl ethanoate and water. The compound formed by the reaction of an alcohol with carboxylic acid is known as ester (fruity smelling compound) and the reaction is called esterification.
USES OF ETHANOL
♦ As an anti-freeze in automobile radiators.
♦ As a preservative for biological specimen.
♦ As an antiseptic to sterilize wounds in hospitals.
♦ As a solvent for drugs, oils, fats, perfumes, dyes, etc.
♦ In the preparation of methylated spirit (mixture of 95% of ethanol and 5% of methanol), rectified spirit (mixture of 95.5% of ethanol and 4.5% of water), power alcohol (mixture of petrol and ethanol) and denatured sprit (ethanol mixed with pyridine).
♦ In cough and digestive syrups.
EVIL EFFECTS OF CONSUMING ALCOHOL
♦ If ethanol is consumed, it tends to slow down metabolism of our body and depresses the central nervous system.
♦ It causes mental depression and emotional disorder.
♦ It affects our health by causing ulcer, high blood pressure, cancer, brain and liver damage.
♦ Nearly 40% accidents are due to drunken drive.
♦ Unlike ethanol, intake of methanol in very small quantities can cause death.
♦ Methanol is oxidized to methanal (formaldehyde) in the liver and methanal reacts rapidly with the components of cells.
♦ Methanal causes the protoplasm to get coagulated, in the same way an egg is coagulated by cooking. Methanol also affects the optic nerve, causing blindness.
ETHANOIC ACID (CH3COOH)
Ethanoic acid is most commonly known as acetic acid and belongs to a group of acids called carboxylic acids. Acetic acid is present in many fruits and sour taste of fruits is because of this acid.
PREPARATION OF ETHANOIC ACID
Ethanol on oxidation in the presence of alkaline potassium permanganate or acidified potassium dichromate gives ethanoic acid.
PROPERTIES OF ETHANOIC ACID
PHYSICAL PROPERTIES
(i) Ethanoic acid is a colourless liquid and has a sour taste.
(ii) It is miscible with water in all proportions.
(iii) Boiling point (391 K) is higher than corresponding alcohols, aldehydes and ketones.
(iv) On cooling, pure ethanoic acid is frozen to form ice like flakes. They look like glaciers, so it is called glacial acetic acid.
CHEMICAL PROPERTIES
(i) Ethanoic acid is a weak acid but it turns blue litmus to red.
(ii) Reaction with metal
Ethanoic acid reacts with metals like Na, K, Zn, etc to form metal ethanoate and hydrogen gas.
(v) Decarboxylation (Removal of CO2)
When sodium salt of ethanoic acid is heated with soda lime (Solid mixure of 3 parts of NaOH and 1 part of CaO) methane gas is formed.
USES OF ETHANOIC ACID
♦ For making vinegar which is used as a preservative in food and fruit juices.
♦ As a laboratory reagent.
♦ For coagulating rubber from latex.
♦ In the preparation of dyes, perfumes and medicine.
INTEXT QUESTIONS PAGE NO. 74
Q1. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Ans: Sodium bicarbonate test (NaHCO3 test)
Alcohol + NaHCO3 → No effervescence
Acid + NaHCO3 → Brisk effervescence
The sample which produces brisk effervescence when treated with NaHCO3 due to release of CO2 is a carboxylic acid.
Q2. What are oxidising agents?
Ans: Those substances which are capable of providing oxygen to other substances are called oxidising agents. e.g., alk. KMnO4 and acidified K2Cr2O7 can both behave as oxidising agents.
SOAPS AND DETERGENTS
Most dirt is oily in nature and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic-end of soap dissolves in water while the carbon chain dissolves in oil. The soap molecules, thus form structures called micelles (see the below figure) where one end of the molecules is towards the oil droplet while the ionic-end faces outside. This forms an emulsion in water. The soap micelle thus helps in dissolving the dirt in water and we can
wash our clothes clean.
MICELLES
Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle.
Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away. The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
INTEXT QUESTIONS PAGE NO. 76
Q1. Would you be able to check if water is hard by using a detergent?
Ans: Detergents are ammonium or sulphonate salts of long chain carboxylic acids. Unlike soap, they do not react with calcium and magnesium ions present in hard water to form scum. They give a good amount of lather irrespective of whether the water is hard or soft. This means that detergents can be used in both soft and hard water. Therefore, it cannot be used to check whether the water is hard or not.
Q2. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Ans: A soap molecule has two parts namely hydrophobic and hydrophilic. With the help of these, it attaches to the grease or dirt particle and forms a cluster called micelle. These micelles remain suspended as a colloid. To remove these micelles (entrapping the dirt), it is necessary to agitate clothes.
EXERCISE QUESTIONS PAGE NO. 77 and 78
Q1. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Ans:
(b) Structure of C2H6 is
It is clear that it has 7 covalent bonds.
Q2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Ans: (c) In butanone, the function group is ketone (one)
Q3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Ans: (b) The unburnt particles of the fuel present in smoke blacken the vessel from outside.
Q4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Ans: Atomic number of
C = 6; H = 1; Cl = 17
Electronic configuration
Q5. Draw the electron dot structures for
(a) ethanoic acid.
(b) H2S.
(c) propanone.
(d) F2 .
Ans:
(a) ethanoic acid.
Q6. What is an homologous series? Explain with an example.
Ans: A homologous series is a series of carbon compounds that have different numbers of carbon atoms but contain the same functional group.
Example of homologous series
Alkane series CnH2n + 2
CH4 Methane, C2H6 Ethane
C3H8 Propane, C4H10 Butane C5H12 Pentane
It can be noticed that there is a difference of −CH2 unit between each successive compound.
Q7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Ans:
I. Distinction based on physical properties
1. Smell Ethanoic acid has a pungent smell. Ethanol has a pleasant smell.
2. Melting point Ethanol has lower melting point (150 K) than ethanoic acid (290 K).
3. Physical state Ethanoic acid is solid (glacial acetic acid) in winters but ethanol is always a liquid.
II. Distriction based on chemical properties
(i) Action with sodium hydrogen carbonate On adding a small amount of sodium hydrogen carbonate to ethanoic acid, carbon dioxide gas is evolved with brisk effervescence. However, no such reaction noticed in case of ethanol.
CH3CHOOH + NaHCO3 → CH3COONa + CO2 ↑ + H2O
(ii) Action with caustic alkalies Ethanoic acids reacts with both sodium hydroxide (NaOH) and potassium hydroxide (KOH) to form corresponding salt and water. Ethanol fails to react with either of these.
CH3CHOOH + NaOH → CH3COONa + H2O
CH3CHOOH + KOH → CH3COOK + H2O
Q8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Ans: A soap is a sodium or potassium salt of long chain fatty acids. It has one polar end and one non-polar end. The polar end is hydrophilic in nature i.e., this end is attracted towards water.
The non-polar end is hydrophobic but lipophilic, i.e., it is attracted towards hydrocarbons.
When soap is added to water, soap molecules arrange themselves in a cluster to keep the nonpolar portion out of water such that the non-polar ends are in the interior of the cluster and the polar ends are on the surface of the cluster. Since the dirt present on clothes is organic in nature and insoluble in water, the hydrophobic ends of the clusters attach themselves to the dirt. This cluster formation in which the dirt is entrapped is the micelle. Micelle formation does not occur in alcohol because the alkyl chain of soap becomes soluble in alcohol.
Q9. Why are carbon and its compounds used as fuels for most applications?
Ans: Carbon burns in oxygen (air) to form carbon dioxide and water.
During this reaction a large amount of heat and light are released. Further, once ignited carbon and its compounds keep on burning without the requirement of additional energy. Hence, they are used as fuels.
C + O2 → CO2 + heat + light
Q10. Explain the formation of scum when hard water is treated with soap.
Ans: Soap does not work properly when the water is hard. A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
Reaction taking place are shown below.
Q11. What change will you observe if you test soap with litmus paper (red and blue)?
Ans: Since soap is basic in nature, it will turn red litmus blue. However, the colour of blue litmus will remain blue.
Q12. What is hydrogenation? What is its industrial application?
Ans: Hydrogenation is the process of addition of hydrogen. Unsaturated hydrocarbons are added with hydrogen in the presence of palladium and nickel catalysts to give saturated hydrocarbons.
This reaction is applied in the hydrogenation of vegetables oils, which contain long chains of unsaturated carbons.
Q13. Which of the following hydrocarbons undergo addition reactions:
C2H6, C3H8, C3H6, C2H2 and CH4.
Ans: Unsaturated hydrocarbons containing double/ triple bond undergo addition reactions.
So, C3H6 and C2H2 will undergo addition reactions.
Q14. Give a test that can be used to differentiate chemically between butter and cooking oil.
Ans: Butter contains saturated compounds while cooking oil contains unsaturated compounds. Since unsaturated compounds are oxidised by alkaline KMnO4 with disappearance of its pink colour.
∴ When cooking oil is treated with a few drops of alkaline KMnO4, pink colour of KMnO4 disappears. With butter however, the pink colour KMnO4 does not disappear.
Q15. Explain the mechanism of the cleaning action of soaps.
Ans: Cleansing action of soaps:
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.
Please click on below link to download CBSE Class 10 Chemistry Carbon And Its Compounds Worksheet Set E
| CBSE Class 10 Biology Our Environment Worksheet Set A |
| CBSE Class 10 Biology Our Environment Worksheet Set B |
| CBSE Class 10 Biology Our Environment Worksheet Set C |
Chapter 04 Carbon and Its Compounds CBSE Class 10 Science Worksheet
The above practice worksheet for Chapter 04 Carbon and Its Compounds has been designed as per the current syllabus for Class 10 Science released by CBSE. Students studying in Class 10 can easily download in Pdf format and practice the questions and answers given in the above practice worksheet for Class 10 Science on a daily basis. All the latest practice worksheets with solutions have been developed for Science by referring to the most important and regularly asked topics that the students should learn and practice to get better scores in their examinations. Studiestoday is the best portal for Printable Worksheets for Class 10 Science students to get all the latest study material free of cost. Teachers of studiestoday have referred to the NCERT book for Class 10 Science to develop the Science Class 10 worksheet. After solving the questions given in the practice sheet which have been developed as per the latest course books also refer to the NCERT solutions for Class 10 Science designed by our teachers. After solving these you should also refer to Class 10 Science MCQ Test for the same chapter. We have also provided a lot of other Worksheets for Class 10 Science which you can use to further make yourself better in Science.
You can download the CBSE Practice worksheets for Class 10 Science Chapter 04 Carbon and Its Compounds for the latest session from StudiesToday.com
Yes, the Practice worksheets issued for Chapter 04 Carbon and Its Compounds Class 10 Science have been made available here for the latest academic session
There is no charge for the Practice worksheets for Class 10 CBSE Science Chapter 04 Carbon and Its Compounds you can download everything free
Regular revision of practice worksheets given on studiestoday for Class 10 subject Science Chapter 04 Carbon and Its Compounds can help you to score better marks in exams
Yes, studiestoday.com provides all the latest Class 10 Science Chapter 04 Carbon and Its Compounds test practice sheets with answers based on the latest books for the current academic session

