Revision Notes on Surface Chemistry:
Adsorption
Reversible and irreversible adsorption
The adsorption is reversible, if the adsorbent can be easily removed from the surface of the adsorbent by physical methods. It is called irreversible adsorption, if the adsorbate cannot be removed from the surface of the adsorbent.
A gas adsorbed on a solid surface can be completely removed in vacuum. It is, therefore, reversible adsorption. Examples of irreversible adsorption are adsorption of oxygen on tungsten adsorbate and adsorption of CO on tungsten surface
Adsorbent, Adsorbate and Interface
• The substances upon whose surface the change of concentration occurs, is called absorbent.
• The substance taken up on the surface is call adsorbate.
• The common surface between the two phases where the adsorbed molecules concentrate is called the interface.
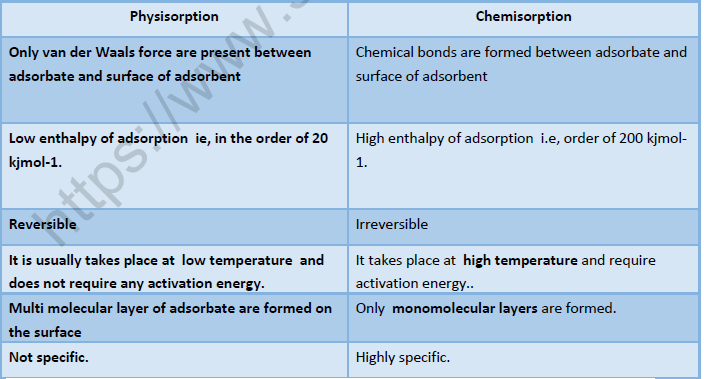
Physisorption and Chemosorption:
Langmular Isotherm:
If A, B & AB represents the adsorbed, absorbent and the absorbed – adsorbent complex then,
A + B ↔AB
ka = Equilibrium constant for adsorption = [AB]/[A][B]
kd = Equilibrium constant for desorption = [A][B]/[AB]
K = Distribution coefficient = ka/kb
Θ = Fraction of the surface of adsorbent available for adsorption.
P = pressure
So,
Θ= KP/(1+KP) (Langmular Equation)
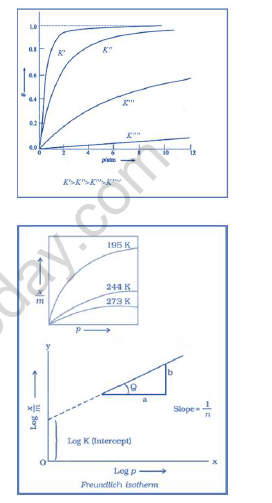
Freundlich Isotherm:
x= Mass of the gas adsorbed
m = Mass of absorbent
p = Pressure
K and n = constants
x/m =k.p(1/n) [n >1]
or
log x/m = log k + 1/n log P
Factors Affecting Adsorption:
• Temperature: An increase of temperature leads to a decrease in amount adsorbed and vice – versa.
• Pressure or concentration: It has been found that in most cases, the adsorption is reversible and an increased pressure of a gases vapour or an increase in concentration of a solute causes increased adsorption.
• Nature of Adsorbate and Adsorbent: The amount of the gas adsorbed depends upon the nature of adsorbent and the gas (adsorbate), which is to be adsorbed. It has been found that easily liquifiable gases such as NH3, HCl, Cl2 , SO2 CO2 etc. are more readily adsorbed than so the called permanent gases such as O2,N2, H2 etc. This is because that molecules of the former type of gases
have greater Vander waal’s or molecular force of attraction.
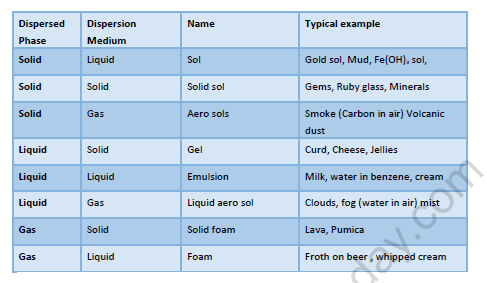
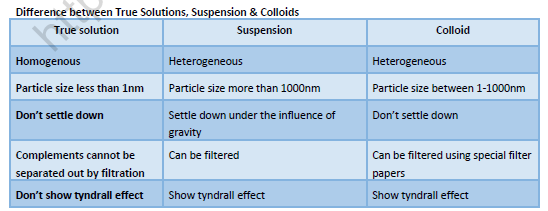
Colloids
Dispersed Phase:
The phase which is dispersed or scattered through the dispersion medium is called Dispersed phase or discontinuous phase.
Dispersion Medium:
The phase in which the scattering is done is called the dispersion medium or continuous medium.
Lyophobic and Lyophilic Colloids:
Those substance whose colloidal solution cannot be prepared by bringing them in contact with a solvent are called Lyophobic (disliking, fearing or hating a liquid). On the other hand those substances whose colloidal solutions can be prepared by bringing them in contact with a liquid solvent are called lyophilic colloids (loving a solvent).
Emulsions:
• Emulsion of oil in water: Those emulsions in which the dispersed phase is oil and water is the dispersion medium. These emulsion are generally represented as O in W emulsions. Examples are milk, vanishing cream etc.
• Emulsions of water in oil: Those emulsion in which the dispersed phase is water while oil is the dispersion medium. These emulsion are generally represented as W in O emulsions. Examples are butter, ice cream etc.
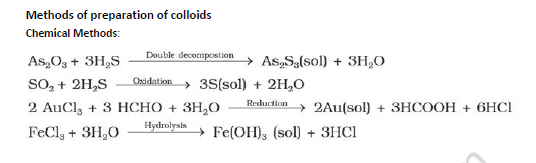
Bredig's method:
An electric arc is struck between two metallic electrodes immersed in dispersion medium. The arc produced vapourises the metal which on further condensation produces particles of colloidal size.
• Peptization:
Process of converting a precipitate into colloidal sol by shaking it with electrolyte in dispersion medium.
Hardy Schulze Rule:
• Ion carrying charge opposite to the colloidal particle has capacity to coagulate the colloid.
• Greater the valency of ion, greater will be the coagulating power.
• Gold Number: The minimum amount of lyophilic colloid in milligrams which can prevent the coagulation of 10 ml gold sol against 1 ml of 10% NaCl solution.
Surfactants
substances which gets preferentially adsorbed at the air – water and solid – water interfaces forming an oriented monolayer where the hydrophilic groups point towards the aqueous phase and the hydrocarbon chain point towards the air or towards the oil phase.
• Anionic surfactants : NSodium salts of higher fatty acids such as sodium palmitate (C15H31COONa), sodium stereate (C17H35COONa) and sodium Oleate (C17H33COONa).
•Catiuonic Surfactants: Those which dissociates in water to yield positively charged ions examples: C18H37 , C16H33(CH3)3 etc.
•Non ionogenic: Those whose molecules cannot undergo dissociation when an alcohol having a higher molecular weight reacts with several molecules of ethylene oxide, a non – ionogenic surfactant is produced.
Please click the link below to download pdf file of NEET Chemistry Surface Chemistry Revision Notes

