Isolation of Metals
General Principles & Processes of Isolation of Metals
Types of Ores:
Ores may be divided into four groups
• Native Ores: These ores contain the metal in free state eg. Silver gold etc. These are usually formed in the company of rock or alluvial impurities like clay, sand etc.
• Oxidised Ores: These ores consist of oxides or oxysalts (eg. carbonates, phosphate) and silicate of metal. Important oxide ore includes, Fe2O3, Al2O3.2H2O etc. and important cabonate ores are limestone (CaCO3), Calamine (ZnCO3) etc.
• Sulphurised Ores: These ores consist of sulfides of metals like iron, lead, mercury etc. Examples
are iron pyrites (FeS2). galena (PbS), Cinnabar (HgS)
• Halide ores: Metallic halides are very few in nature. Chlorides are most common examples include horn silver (AgCl) carnallite KCl. MgCl2.6H2O and fluorspar (CaF2) etc.
Metallurgy:
It is the process of extracting a metal from its ores. The following operations are carried out for obtaining the metal in the pure form.
• Crushing of the ore
• Dressing or concentration of the ore.
• Reduction of metal.
• Purification or refining of the metal
Concentration
Physical Method
Gravity separation: The powdered ores is agitated with water or washed with a running stream of water. The heavy ore particles of sand, clay etc. are washed away.
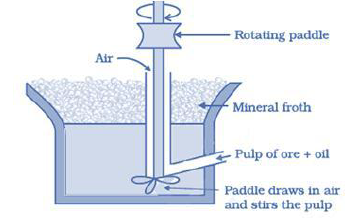
Froth Floatation Process: The finely divided ore is introduced into water containing small quantity of oil (e.g. Pine Oil). The mixture is agitated violently with air a froth is formed which carries away along with it the metallic particles on account of the surface tension forces. The froth is transferred to another bath where gangue-free ore settles down.
Electro Magnetic Separator: A magnetic separator consists of a belt moving over two rollers, one of which is magnetic. The powdered ore is dropped on the belt at the other end. Magnetic portion of the ore is attracted by the magnetic roller and falls near to the roller while the non-magnetic impurity falls farther off
Chemical Methods
Calcination: Carbonate or hydrated oxide ores are subjected to the action of heat in order of expel water from hydrated oxide and carbon dioxide from a carbonate.
Examples:
ZnCO3 --> ZnO + CO2
CaCO3 --> CaO + CO2
Al2O3×2H2O --> Al2O3 + 2H2O
2Fe2O3×3H2O --> 2Fe2O3 + 3H2O
Roasting: Sulphide ores either are subjected to the action of heat and air at temperatures below their melting points in order to bring about chemical changes in them.
Examples:
2PbS + 3O2 --> 2PbO + 2SO2
PbS + 2O2 --> PbSO4
2ZnS + 3O2 --> 2ZnO + 2SO2
ZnS + 2O2 --> ZnSO4
CuS + 2O2 --> CuSO4
2Cu2S + 3O2 --> 2Cu2O + 2SO2
Leaching: It involves the treatment of the ore with a suitable reagent as to make it soluble while impurities remain insoluble. The ore is recovered from the solution by suitable chemical method.
Al2O3 + 2NaOH -->2 NaAlO2 + H2O
Reduction of Free Metal:
Smelting:
Reduction of a metal from its ore by a process involving melting Several reducing agents such as sodium, magnesium and aluminium are used for reduction.
The calcinated or roasted ore is mixed with carbon (coal or coke) and heated in a reverberatory or a blast furnace.
Carbon and carbon monoxide produced by incomplete combustion of carbon reduce the oxide to the metal.
Flux:
The ores even after concentration contain some earthy matter called gangue which is heated combine with this earthy matter to form an easily fusible material. Such a substance is known as flux and the fusible material formed during reduction process is called slag.
• Acidic fluxes like silica, borax etc are used when the gangue is basic such as lime or other metallic oxides like MnO, FeO, etc
• Basic fluxes like CaO, lime stone (CaCO3), magnesite (MgCO3), hematite (Fe2O3) etc are used when the gangue is acidic like silica, P4O10 etc.
Refining
The metals obtained by the application of above reduction methods from the concentration ores are usually impure. The impure metal is thus subjected to some purifying process known as refining in order to remove undesired impurities. Various process for this are
a) Liquation process
b) Distillation process
c) Cupellation
d) Poling
e) Electrolytic refining
f) Bessemerisation
Thermodynamic Principles of Metallurgy:
ΔG =ΔH-TS
or ΔG0 =-RT ln K
An element A can reduce element B if ΔG value for oxidation of A to AO is lower than the ΔG value for oxidation of B to BO.
i.e. ΔG(A→AO) < ΔG(B→BO)
Please click the link below to download pdf file of NEET Chemistry Isolation of Metals Revision Notes

