Refer to BITSAT Physics Atoms MCQs provided below available for download in Pdf. The MCQ Questions for Full Syllabus Physics with answers are aligned as per the latest syllabus and exam pattern suggested by BITSAT, NCERT and KVS. Atoms Full Syllabus MCQ are an important part of exams for Full Syllabus Physics and if practiced properly can help you to improve your understanding and get higher marks. Refer to more Chapter-wise MCQs for BITSAT Full Syllabus Physics and also download more latest study material for all subjects
MCQ for Full Syllabus Physics Atoms
Full Syllabus Physics students should refer to the following multiple-choice questions with answers for Atoms in Full Syllabus.
Atoms MCQ Questions Full Syllabus Physics with Answers
Question: Hydrogen (H), deuterium (D), singly ionized helium (He+) and doubly ionized lithium (Li++) all have one electron around the nucleus. Consider n = 2 to n = 1 transition. The wavelengths of emitted radiations are λ1, λ2, λ3 and λ4 respectively. Then approximately:
- a) λ1 = λ2 = 4 λ3 = 9 λ4
- b) 4 λ1 = 2λ2 = 2 λ3 = λ4
- c) λ1 = 2 λ2 = 2√2 λ3 = 3√2 λ4
- d) λ1 = λ2 = 2 λ3 = 3√2 λ4
Answer: λ1 = λ2 = 4 λ3 = 9 λ4
Question: If the series limit wavelength of Lyman series for the hydrogen atom is 912 Å, then the series limit wavelength for Balmer series of hydrogen atoms is
- a) 912 Å
- b) 912 × 2 Å
- c) 912 × 4 Å
- d)
Answer: 912 × 4 Å
Question: One of the lines in the emission spectrum of Li2+ has the same wavelength as that of the 2nd line of Balmer series in hydrogen spectrum. The electronic transition corresponding to this line is n = 12→ n = x. Find the value of x.
- a) 8
- b) 6
- c) 7
- d) 5
Answer: 6
Question: Energy required for the electron excitation in Li++ from the first to the third Bohr orbit is
- a) 36.3 eV
- b) 108.8 eV
- c) 122.4 eV
- d) 12.1 eV
Answer: 108.8 eV
Question: The angular momentum of electron in nth orbit is given by
- a) nh
- b)
- c)
- d)
Answer:
Question: The energy of electron in the nth orbit of hydrogen atom is expressed as 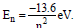 The shortest and longest wavelength of Lyman series will be
The shortest and longest wavelength of Lyman series will be
- a) 910 Å, 1213 Å
- b) 5463 Å, 7858 Å
- c) 1315 Å, 1530 Å
- d) None of these
Answer: 910 Å, 1213 Å
Question: If the Kα radiation of Mo (Z = 42) has a wavelength of 0.71 Å, calculate wavelength of the corresponding radiation of Cu, i.e., Kα for Cu (Z = 29) assuming σ= 1.
- a) 1.52 Å
- b) 5.14 Å
- c) 3.02 Å
- d) 0.52 Å
Answer: 1.52 Å
Question: The energies of energy levels A, B and C for a given atom are in the sequence EA < EB < EC. If the radiations of wavelengths λ1, λ2 and λ3 are emitted due to the atomic transitions C to B, B to A and C to A respectively then which of the following relations is correct ?
- a) λ1 + λ2 +λ3 = 0
- b) λ3 = λ1 2 + λ2
- c) λ3 = λ1 + λ2
- d)
Answer:
Question: The third line of Balmer series of an ion equivalnet to hydrogen atom has wavelength of 108.5 nm. The ground state energy of an electron of this ion will be
- a) 3.4 eV
- b) 13.6 eV
- c) 54.4 eV
- d) 122.4 eV
Answer: 54.4 eV
Question: Hydrogen atom in ground state is excited by a monochromatic radiation of λ = 975 Å. Number of spectral lines in the resulting spectrum emitted will be
- a) 3
- b) 2
- c) 6
- d) 10
Answer: 6
Question: Taking Rydberg’s constant RH = 1.097 × 107m, first and second wavelength of Balmer series in hydrogen spectrum is
- a) 2000 Å, 3000 Å
- b) 1575 Å, 2960 Å
- c) 6529 Å, 4280 Å
- d) 6552 Å, 4863 Å
Answer: 6552 Å, 4863 Å
Question: In which of the following series, does the 121.5nm line of the spectrum of the hydrogen atom lie?
- a) Lyman series
- b) Balmer series
- c) Paschen series
- d) Brackett series.
Answer: Lyman series
Question: The ionisation potential of H-atom is 13.6 V. When it is excited from ground state by monochromatic radiations of 970.6 Å, the number of emission lines will be (according to Bohr’s theory)
- a) 10
- b) 8
- c) 6
- d) 4
Answer: 6
Question: The angular momentum of an electron in first orbit of Li++ ion is –
- a)
- b)
- c)
- d)
Answer:
Question: The de Broglie wavelength of the electron in the first Bohr orbit of the hydrogen atom is
- a) Equal to the diameter of the first orbit
- b) Equal to the circumference of the first orbit
- c) Equal to half the circumference of the first orbit
- d) Independent of the size of the first orbit
Answer: Equal to the circumference of the first orbit
MCQs for Atoms Physics Full Syllabus
Expert teachers of studiestoday have referred to NCERT book for Full Syllabus Physics to develop the Physics Full Syllabus MCQs. If you download MCQs with answers for the above chapter you will get higher and better marks in Full Syllabus test and exams in the current year as you will be able to have stronger understanding of all concepts. Daily Multiple Choice Questions practice of Physics will help students to have stronger understanding of all concepts and also make them expert on all critical topics. After solving the questions given in the MCQs which have been developed as per latest books also refer to the NCERT solutions for Full Syllabus Physics. We have also provided lot of MCQ questions for Full Syllabus Physics so that you can solve questions relating to all topics given in each chapter. After solving these you should also refer to Full Syllabus Physics MCQ Test for the same chapter.
You can download the BITSAT MCQs for Full Syllabus Physics Atoms for latest session from StudiesToday.com
Yes, the MCQs issued by BITSAT for Full Syllabus Physics Atoms have been made available here for latest academic session
You can find BITSAT Full Syllabus Physics Atoms MCQs on educational websites like studiestoday.com, online tutoring platforms, and in sample question papers provided on this website.
To prepare for Atoms MCQs, refer to the concepts links provided by our teachers and download sample papers for free.
Yes, there are many online resources that we have provided on studiestoday.com available such as practice worksheets, question papers, and online tests for learning MCQs for Full Syllabus Physics Atoms

