I. Text book question and Answer:
1. What are transition metals? Give four examples
Transition metal as an element whose atom has an incomplete d sub shell or which can give rise to cations with an incomplete d sub shell. Example : Iron, Cobalt, Nickel, Copper.
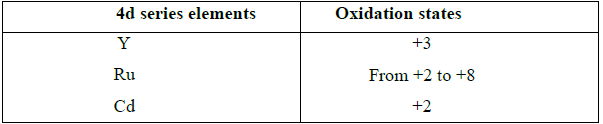
2. Explain the oxidation states of 4d series elements.
• At the beginning of the series, +3 oxidation state is stable but towards the end +2 oxidation state become stable.
• Hence the first and last elements show less number of oxidation states and the middle elements with more number of oxidation states.
3. What are inner transition elements?
• The elements in which the extra electron enters (n-2)f orbitals are called f-block elements.
• These elements are also called as inner transition elements because they form a transition series within the transition elements.
• In the inner transition elements there are two series of elements.
1) Lanthanoids (Previously called lanthanides)
2) Actinoids (Previously called actinides)
4. Justify the position of lanthanides and actinides in the periodic table.
• The actual position of Lanthanoids in the periodic table is at group number 3 and period number6.
• In sixth period after lanthanum, the electrons are preferentially filled in inner 4f sub shell and these fourteen elements following lanthanum show similar chemical properties. Similarly, the fourteen elements following actinium resemble in their physical and chemical properties.
• Therefore these elements are grouped together and placed at the bottom of the periodic table.
• This position can be justified as follows.
1. Lanthanoids have general electronic configuration [Xe]4f1-14 5d0-16s2
2. The common oxidation state of Lanthanoids is+3
3. All these elements have similar physical and chemical properties. Hence a separate position is provided to the inner transition elements.
5. What are actinides? Give three examples.
The fourteen elements following actinium, i.e. from Thorium (Th) to Lawrentium (Lr) are called actinoids.
Examples : Thorium (Th), Uranium (U) and Plutonium (Pu)
6. Why Gd3+ is colourless?
Electronic configuration of Gd is [Xe]4f75d16s2
Electronic configuration of Gd3+ is [Xe] 4f75d06s0
In Gd3+, no electrons are there in outer 5d orbitals.
d-d transition is not possible. So Gd3+ is colourless.
7. Explain why compounds of Cu2+ are coloured but those of Zn2+ are colourless.
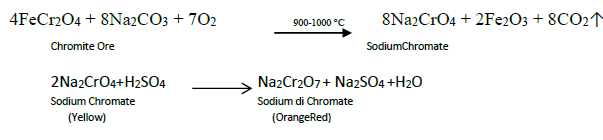
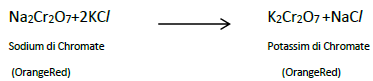
8. Describe the preparation of potassiumdichromate
Ore : Chromite Ore
Concentration : Gravity Separation
9. What is lanthanoid contraction and what are the effects of lanthanoid contraction?
As we move across 4f series, the atomic and ionic radii of lanthanoids show gradual decrease with increase in atomic number. This decrease in ionic size is called lanthanoid contraction.
Consequences of lanthanoid contraction:
1) As we move from Ce3+ to Lu3+, the basic character of Ln3+ ions decreases. Due to the decrease in the size of Ln3+ ions, the ionic character of Ln-OH bond decreases (covalent character increases) which results in the decrease in the basicity.
2) Because of this very small change in radii of lanthanoids, their chemical properties are quite similar.
3) The elements of the second and third transition series resemble each other more closely than the elements of the first and second transition series.
10. Complete the following:
11. What are interstitial compounds?
An interstitial compound or alloy is a compound that is formed when small atoms like hydrogen, boron, carbon or nitrogen are trapped in the interstitial holes in a metal lattice. Example : TiC, ZrH1.92,Mn4N
12. Calculate the numbers of unpaired electrons in Ti3+, Mn2+ and calculate the spin only magnetic moment.
13 Write the electronic configuration of Ce4+ and Co2+
Electronic Configuration of Ce4+ = [Xe] 4f 05d06s0
Electronic configuration of Co2+ = [Ar]3d74s0
14. Explain briefly how +2 states become more and more stable in the first half of the first row transition elements with increasing atomic number.
E0 M2+/M for 3d series upto Mn is highly negative. Therefore +2 states become more stable in the first half of the first row transition elements.
15. Which is more stable? Fe3+ or Fe2+ -Explain.
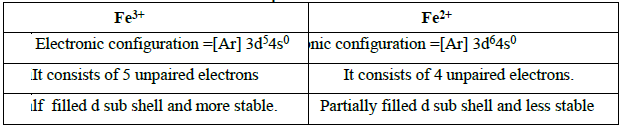
Hence Fe3+ is more stable than Fe2+.
16. Explain the variation EOM3+/M2+in 3dseries
• The standard electrode potential for the M3+/M2+ half cell gives the relative stability between M3+ and M2+.
• The negative values for titanium, vanadium and chromium indicate that the higher oxidation state is preferred. If we want to reduce such a stable Cr3+ ion, strong reducing agent which has high negative value for reduction potential like metallic zinc (E=- 0.76V) is required.
• The high reduction potential of Mn3+ / Mn2+ indicates Mn2+ is more stable than Mn3+. For Fe3+ / Fe2+ the reduction potential is 0.77V, and this low value indicates that both Fe3+ and Fe2+ can exist under normal conditions.
17. Compare lanthanides and actinides.
18. Explain why Cr2+ is strongly reducing while Mn3+ is strongly oxidizing.
Cr2+ is reducing as its configuration changes from d4 to d3, the latter having a half filled t2g level. On the other hand, the change from Mn2+ to Mn3+ results in the half – filled (d5) configuration which has extra stability.
19. Compare the ionization enthalpies of first series of the transition elements.
Ionization energy of transition elements is intermediate between those of s and p block elements. As we move from left to right in a transition metal series, the ionization enthalpy increases as expected. This is due to increase in nuclear change corresponding to the filling of d electrons.
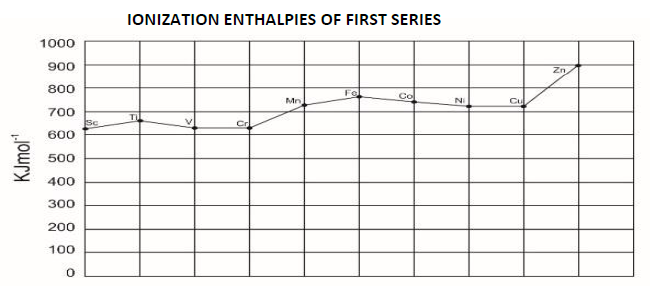
20. Actinoid contraction is greater from the elements to element than the lanthanoid contraction, why?
This is due to poor shielding effect by 5f electrons in actinoids as compared to that by 4f electrons in lanthanoids.
21. Out of Lu(OH)3and La(OH)3which is more basic and why?
22. Why Europium (II) is more stable than Cerium(II)?
23. Why do Zirconium and Hafnium exhibit similar properties?
Zirconium and Hafnium exhibit similar properties due to lanthanoid contraction.
24. Which is stronger reducing agent Cr2+ or Fe2+?
If the standard electrode potential (EO) of a metal is large and negative, the metal is a powerful reducing agent, because it loses electrons easily.
Since Cr2+ has larger negative value of E0, Cr2+ is a stronger reducing agent than Fe2+.
25. The 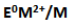
value for copper is positive. Suggest a possible reason for this.
E0 M2+/M for any metal depends upon the sum of the enthalpy changes taking place in the following steps.
Copper possesses a high enthalpy of atomization and low enthalpy of hydration. Hence E0Cu2+/Cu is positive.
26. Predict which of the following will be coloured in aqueous solutions Ti2+, V3+,Ti3+, Cu+, Sc3+, Fe3+, Ni2+ and Co3+
27. Describe the variable oxidation state of 3d series elements.
The first transition metal scandium exhibits only +3 oxidation state, but all other transition elements exhibit variable oxidation states by losing electrons from (n-1)d orbital and ns orbitals as the energy difference between them is very small.
The first and last elements show less number of oxidation states and the middle elements with more number of oxidation states.
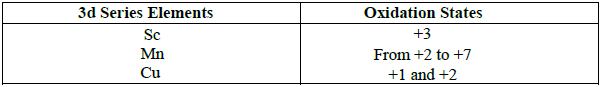
28. Which metal in the 3d series exhibits +1 oxidation state most frequently and why?
Copper exhibits +1 oxidation state in the 3d series.
Reason : Electronic configuration of Cu = [Ar] 3d104s1
It can easily lose 4s1 electron to give stable 3d10 configuration. Hence, it exhibits +1 oxidation state.
29. Why first ionization enthalpy of chromium is lower than that of zinc?
30. Transition metals show high melting points.Why?
The high melting points of transition metals are attributed to the involvement of greater number of electrons in the inter atomic metallic bonding from (n-1)d electrons in addition to ns electrons.
II Evaluate yourself
1. Why iron is more stable in +3 oxidation state than in +2 and the reverse is true for manganese?
Electronic Configuration.
Fe3+ ion has half filled d orbital which is more sable than partially filled d orbital of Fe2+ Mn2+ ion has half filled d orbital which is more stable than partially filled d orbital of Mn3+
2. Compare the stability of Ni4+ and Pt4+ from their ionization enthalpy values
Pt4+ is thermodynamically more stable than Ni4+ .Smaller the Ionisation enthalpy greater will be the thermodynamic stability of its compounds
III.ADDITIONAL QUESTIONS
1. How d block elements are classified?
i. 3d series (4th period) -Scandium to Zinc
ii. 4d series (5th period) - Yttrium to Cadmium
iii. 5d serried (6th period) - Lanthanum,Haffinium to Mercury
iv. 6d series (7th period) - Actinium, Rutherfordium to Copernicium.
2.Which transition series contains radioactive elements?
6dseries(7th period) Ac,Rf to Cn.
In this period all the elements are radioactive and have very low half –life period.
3.Write the electronic configuration of Cr and Cu
Cr : Z=24
Cr : [Ar] 3d54s1
Cu : Z=29
Cu :[Ar] 3d104s1
4.a In transition metals, which group elements are not hard?
b. Which metal has highest electrical conductivity at room temperature.
a. Group 11 elements are not hard
b. Silver
5 Give reason for the slight increase in atomic radius of Zn
The d orbitals of Zn contain 10 electrons in which the repulsive interaction between the electrons is more than the effective nuclear charge and hence the orbitals slightly expand and atomic radius slightly increases.
6. Ionisation enthalpy to form Ni2+ =2490kJ
Ionization enthalpy to form Pt2+ =2655kJ
Which is thermodynamically stable Ni2+ (or) Pt2+? Why?
The energy required to form Ni2+ is less than that of Pt2+ so Ni2+compounds are thermodynamically more stable than Pt2+compounds.
7. Calculate the number of unpaired electrons for the following ions?
a. Cu+ b. Ni2+
Number of unpaired electrons are zero (n=0)
Number of unpaired electrons are two (n = 2)
8. Name the catalyst used for the following reaction?
c. In the manufacture of Sulphuric acid from SO3
a.Co2(CO)8
b.Rh / Ir Complex
c.Vanadium pentoxide (V2O5)
9. Why transition metals form number of alloys ?
Atomic sizes of transition metals are similar and one metal atom can be easily replaced by another metal atom from its crystal lattice to form an alloy.
10. Why transition elements form complexes?
i. Transition metal ions are small and highly charged
ii. They have vacant low energy orbitals to accept an electron pair donated by other group
11. Under what oxidation state do transition metals form ionic oxide and covalent oxides?
Metals in lower oxidation state form ionic oxides.
Metals in higher oxidation state form covalent oxides
Example Mn2O7 is covalent. Oxidation state is +7.
12. Zn, Cd and Hg do not have partially filled d-orbitals why they are treated as transition elements?
They are treated as transition elements because their properties are an extension of the properties of the respective transition elements.
13. When potassium dichromate acts as oxidizing agent in the presence of H+ ion, what is the change in oxidation state of Chromium? Give equation ?
14.What is the action of heat on K 2Cr2 O7?
On heating it decomposes and forms Cr2O3 and molecular oxygen
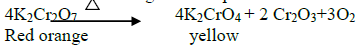
15.How neutral KMnO4 oxidizes thio sulphate ion? Give equation?
It oxidizes thio sulphate into sulphate
16.a. Which element in 3d series does not form ionic metal oxide ?
b. What is the acid formed when Mn2O7 dissolved in water?
a. Scandium
b .Mostly higher oxide are acidic. Mn2O7 dissolved in water to give permanganic acid (HMnO4)
17. Why d orbitals containing symmetrical distribution of electron is more stable?
When the d – Orbitals are considered together, they will constitute a sphere. So the half filled and fully filled configuration leads to complete symmetrical distribution of electron density. An unsymmetrical distribution of electron density will result in building up of a potential difference. To decrease this and to achieve a tension free State with lower energy, a symmetrical distribution is preferred.
18. In first transition series from Sc to V atomic radius decreases, thereafter up to Cu atomic radius nearly the same. Why ?
The added 3d electrons only partially shield the increased nuclear charge and hence the effective nuclear change increases slightly. At the same time the extra electrons added to the 3d sub shell strongly repel the 4s electron These two forces are operated in opposite direction As they tend to balance each other it leads to constancy in atomic radius
19.Which is more acidic in nature MnO(Mn2+) or Mn2O7 (Mn7+) why?
Mn2O7 is more acidic in nature. Acidic strength increases with increase in oxidation state of the element. In higher oxidation state there is no scope for further loss of electron, rather it can accept electrons. So it is more acidic in nature.
20.Classify the following oxides as acidic, basic and amphoteric oxides?
i.CrO ii. Cr2O3 iii.CrO3
i. CrO –Basic oxide
ii.Cr2O3 – Amphoteric oxide
iii. CrO3 – Acidic oxide
21. Calculate spin magnetic moment of Co2+?
22.Why transition metal and its compound act as good catalyst?
Transition metal has energetically available d orbitals.
d-orbitals can accept electrons from reactant molecule and form bond with reactant molecule using its d electron.
23. Draw the structure of chromate ion and dichromate ion ?
24.What is zeigler – Natta Catalyst?Give a reaction in which it catalysis?
zeigler – Natta Catalyst ( TiCl4 + Al (C2H5)3
Mixture of TiCl4 and trialkyl aluminium.
25.a) What is the oxidation state of chromium in chromate and dichromate ion?
b) Which is predominant in alkaline and acidic solution?
a) In both chromate and dichromate ion, oxidation state of chromium is +6
b) In aqueous solution chromate and dichromate ions are inter convertible.
In alkaline solution chromate ion is predominant.
In acidic solution dichoromate ion is predominant.
26.Give the reaction of cold and hot conc H2SO4 with KMnO4?
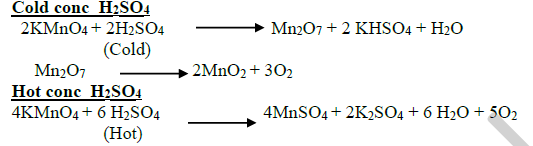
27. Exhibit the oxidizing property of MnO4- ion in acid medium, neutral medium and alkaline medium ?
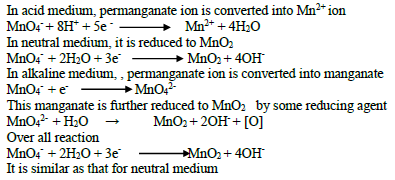
28. HCl and HNO3 are not used for generating acidic medium for KMnO4Why ? Suggest suitable acid medium for KMnO4?
HCl cannot be used. Since It reacts with KMnO4
2MnO4- + 10Cl- + 16H+ → 2Mn2+ + 5Cl2 +8H2O
HNO3 also cannot be used since it is a good oxidizing agent and react with reducing agents in the reaction.
H2SO4 is the most suitable acid since it does not react with potassium permanganate
29. Calculate the equivalent weight of KMnO4 ?
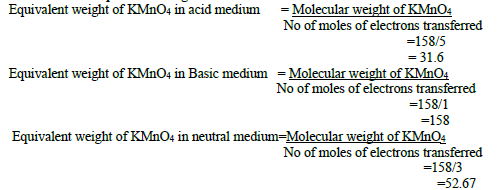
30. Write the uses of KMnO4?
i. It is used as a strong oxidizing agent.
ii. It is used for the treatment of various skin infections and fungal infections of the foot.
iii. It used in water treatment industries to remove iron and hydrogen sulphide from well water.
iv. It is used as a Bayer’s reagent for detecting unsaturation in an organic compound.
v. It is used in quantitative analysis for the estimation of ferrous salts, oxalates, hydrogen peroxide and iodides.
31. Complete the following reaction and give the balanced equation?
32. What is the hybridization in Mn7+ ion of KMnO4 and what is the structure of permanganate ion?
Permanganate ion has tetrahedral geometry in which the central Mn7+ is sp3 hybridised.
33. Compound A is an orange red crystalline solid which on heating with NaCl and conc H2SO4 evolves red orange vapours B. On passing the vapors of B into a solution of NaOH and then adding the solution of acetic acid and lead acetate, yellow precipitate C is obtained. Identify A,B, andC. Give chemical equations for these reactions What is the name of this test?
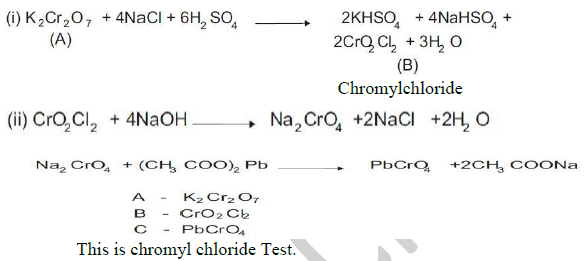
34.What are the properties of Interstitial compound?
They are hard and show electrical and thermal conductivity
i. They have high melting points higher than those of pure metals
ii. Transition metal hydrides are used as powerful reducing agents
iii. Metallic carbides are chemically inert.
35. Complete the following reactions and give the balanced equations?
36. What is Hume- Rothery rule to form a substitute alloy?
i. The difference between the atomic radius of solvent and solute is less than 15%
ii. Both the solvent and solute must have the same crystal structure and valence.
iii. The eletronegativity difference between solvent and solute must be close to zero.
37.What are the uses of potassium dichromate?
i. It is used as a strong oxidizing agent.
ii. It is used in dyeing and printing.
iii.It used in leather tanneries for chrome tanning.
iv.It is used in quantitative analysis for the estimation of iron compounds and iodides.

