LEARNING POINTS:
Units of concentration of Solution :
(i) Mass Percentage (w/w): Amount of solute present in grams dissolved per 100g of solution.
Ex: 10% (w/w) glucose in water by mass, it means that 10 g of glucose is dissolved in 90 g of water resulting in a 100 g solution.
(ii) Volume percentage (v/v): Volume of solute present in 100ml of solution.
Ex : 10%(v/v) Alcohol in water by volume, it means that 10 ml of alcohol present in a 100 ml of solution.
(iii) Parts per million (ppm)- Amount of substance present in grams in 106 gm of solution.
(iv) Mole fraction (X) –It is the ratio of number of mole of a particular component to the total number of moles of all the components present in the solution.
XA = nA / (nA + nB), XB = nB / (nA + nB),
Where X A & X B are the mole fractions of Solvent and Solute respectively.
n A & n B are the number of moles of Solvent and Solute respectively.
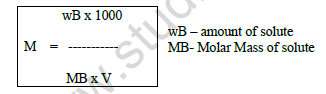
(v) Molarity (M) – No. of mole of solute present per litre of solution.
M = ( nB / V) x 1000
Where , nB – No. of moles of solute
V – Volume of solution is ml.
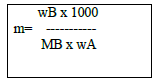
(vi) Molality :- (m) No. of moles of solute present per 1000 g or 1. kg solvent.
Where , nB – No. of moles of solute
wA – amount of solvent in gm.
wB – amount of solute
MB- Molar Mass of solute
Solubility:
1) Solubility of a Solid in Liquids: It is observed that polar solutes dissolve in polar solvents and non polar solutes in nonpolar solvents.
Ex: Sodium chloride and sugar dissolve readily in water.Naphthalene and anthracene dissolve readily in benzene.
In general, a solute dissolves in a solvent if the intermolecular interactions are similar in the two
Dissolution: When a solid solute is added to the solvent, some solute dissolves and its concentration increases in solution. This process is known as dissolution.
Crystallisation: Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is known as crystallisation.
A stage is reached when the two processes occur at the same rate. Under such conditions, number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached.
Solute + Solvent = Solution
Effect of temperature:
The solubility of a solid in a liquid is significantly affected by temperature changes. According to Le Chateliers Principle, the dissolution process is endothermic (Δsol H > 0), the solubility should increase with rise in temperature and if it is exothermic (Δsol H < 0) the solubility should decrease. These trends are also observed experimentally.
Effect of pressure:
Pressure does not have any significant effect on solubility of solids in liquids.
2. Solubility of gases in Liquids:
1) Oxygen dissolves only to a small extent in water. It is this dissolved oxygen which sustains all aquatic life.
2) Hydrogen chloride gas (HCl) is highly soluble in water.
Solubility of gases in liquids is greatly affected by pressure and temperature. The solubility of gases increase with increase of pressure.
Henry was the first to give a quantitative relation between pressure and solubility of a gas in a solvent which is known as Henry’s law:. The law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
The solubility of a gas in a liquid solution is a function of partial pressure of the gas.
If we use the mole fraction of a gas in the solution as a measure of its solubility, then it can be said that “The mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution.”
p = KH x X
Here KH = is the Henry’s law constant.
X = is mole fraction of gas in the solution.
P = partial pressure of the gas.
Henry’s law & its applications:
1) Solubility of a gas increases with decrease of temperature. It is due to this reason that aquatic species are more comfortable in cold waters rather than in warm waters.
2) To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
3) Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater. Increased pressure increases the solubility of atmospheric gases in blood. When the divers come towards surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. This blocks capillaries and creates a medical condition known as bends, which are painful and dangerous to life.
To avoid bends, as well as, the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
4) At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
Effect of Temperature:
Solubility of gases in liquids decreases with rise in temperature. The dissolution of a gas in liquid is an exothermic process involves dynamic equilibrium and thus must follow Le-Chatelier’s Principle. As the temperature increases the solubility of gas decreases.
From Henry’s law:
As the temperature increases the value of KH increases, we know that KH is inversely proportional to Mole fraction of the gas i.e solubility of the gas (From Henry’s law). So, as the temperature increases the solubility of a gas decreases.
Vapour Pressure and Raoult’s law :-
a) Raoult’s law for binary solutions of volatile liquids: At a given temperature, for a solution of volatile liquids, the partial vapour pressure of each component is equal to the product of the vapour pressure of the pure component and its mole fraction.
If the solution contains A & B are two volatile liquids, then
i.e PA = p0A× XA & PB = p0B ×XB
Where → pA and pB are the vapour pressures of A and B in solution respectively.
p0A α p0B are the vapour pressures of A and B in their pure state respectively.
XA and XB are the molefractions of A and B in solution respectively.
b) Raoult’s law for solution containing Non – volatile solute: At a given temperature, the relative lowering vapour pressure of a solution is equal to the mole fraction of the solute.
Derivation :
We know that, from Raoults law,
P solution = pA + pB
P solution = p0A× XA + p0B ×XB
P solution = p0A ×XA + 0 ( Since p0B =0 , because B is anon volatile solute)
P solution = XA p0A
P solution = (1-XB) p0A (Since XA + XB =1)
(P solution - P0A) = - XB p0A
( p0A - P solution) / XB = p0A
Where , ( p0A - P solution) = Lowering of vapour pressure
( p0A - P solution)/ p0A = Relative lowering of vapour pressure.
XB = Mole fraction of Solute.
Please click on below link to download CBSE Class 12 Chemistry Solutions Notes

