Read and download free pdf of CBSE Class 9 Science Structure of The Atom Worksheet Set A. Download printable Science Class 9 Worksheets in pdf format, CBSE Class 9 Science Chapter 4 Structure of the Atom Worksheet has been prepared as per the latest syllabus and exam pattern issued by CBSE, NCERT and KVS. Also download free pdf Science Class 9 Assignments and practice them daily to get better marks in tests and exams for Class 9. Free chapter wise worksheets with answers have been designed by Class 9 teachers as per latest examination pattern
Chapter 4 Structure of the Atom Science Worksheet for Class 9
Class 9 Science students should refer to the following printable worksheet in Pdf in Class 9. This test paper with questions and solutions for Class 9 Science will be very useful for tests and exams and help you to score better marks
Class 9 Science Chapter 4 Structure of the Atom Worksheet Pdf
Question. Select the correct statements.
(i) An atom is divisible and consists of charged particles.
(ii) It was known by 1900 that the atom was not a simple, indivisible particle but contained at least one sub-atomic particle – the electron.
(iii) E. Goldstein in 1886 discovered the presence of new radiations called canal rays which led to the discovery of another sub-atomic particle – the proton.
(iv) Proton had a charge, equal in magnitude but opposite in sign to that of the electron and its mass was approximately 2000 times as that of the electron.
(v) The mass of an electron is considered to be negligible and its charge is minus one.
(a) (i), (ii) and (iii)
(b) (iii), (iv) and (v)
(c) (ii), (iii) and (iv)
(d) All the statements are correct.
Answer. D
Question. Rutherford concluded from the a-particle scattering experiment that
(i) most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
(ii) very few particles were deflected from their path, indicating that the negative charge of the atom occupies very little space.
(iii) a very large fraction of α-particles were deflected by 180°°, indicating that all the negative charge and mass of the gold atom were not concentrated in a very small volume within the atom.
Identify the incorrect statements.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (i), (ii) and (iii)
Answer. B
Question. Which of the following has the same number of electrons as an oxide ion (O2–)?
(a) K+
(b) Mg2+
(c) Cl–
(d) S2–
Answer. B
Question. Select the correct statements.
(i) The radius of the nucleus is about 107 times less than the radius of the atom.
(ii) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
(iii) The electrons revolve around the nucleus in circular paths.
(iv) The size of the nucleus is very large as compared to the size of the atom.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i), (ii) and (iii)
(d) All the statements are correct.
Answer. B
Question. Which pair of molecules has the same number of electrons?
(a) N2 and F2
(b) Cl2 and CO2
(c) H2O and H2S
(d) O2 and C2H4
Answer. D
Question. Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(iii) Bohr’s atomic model
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (i)
(c) (ii), (i) and (iii)
(d) (iii), (ii) and (i)
Answer. C
Question. Dalton’s atomic theory successfully explained
(i) law of conservation of mass
(ii) law of constant composition
(iii) law of radioactivity
(iv) law of multiple proportion.
(a) (i), (ii) and (iii)
(b) (i), (iii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (ii) and (iv)
Answer. D
Question. The main drawback of Rutherford’s model of the atom is that
(a) it does not explain the stability of atom
(b) it does not show the location of protons
(c) it does not explain neutral nature of an atom
(d) it does not explain existence of a nucleus in an atom.
Answer. A
Question. The nucleon number of the bromine atom is 79 and its proton number is 35. Which of the following is true about the bromine atom?
(a) It has 79 neutrons.
(b) It has 44 electrons.
(c) Its electronic configuration contains three shells which has 7 electrons in outermost shell.
(d) It has similar chemical properties as chlorine.
Answer. D
Question. The ion of an element has 3 positive charges.Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16
Answer. B
Question. A monovalent anion has 10 electrons and 10 neutrons. The atomic number and mass number of the element are respectively ______ and _____.
(a) 10, 20
(b) 9, 18
(c) 11, 20
(d) 9, 19
Answer. D
Question. In a sample of ethyl ethanoate (CH3COOC2H5) the two oxygen atoms have the same number of electrons but different number of neutrons.Which of the following is the correct reason for it?
(a) One of the oxygen atoms has gained electrons.
(b) One of the oxygen atoms has gained two neutrons.
(c) The two oxygen atoms are isotopes.
(d) The two oxygen atoms are isobars.
Answer. C
Question. An element L has 9 protons and its valency is 1. Another element M has valency 3 and 5.What is the difference in the number of electrons in L and M?
(a) 6
(b) 5
(c) 4
(d) 3
Answer. A
Question. An atom with 3 protons and 4 neutrons will have a valency of
(a) 3
(b) 7
(c) 1
(d) 4
Answer. C
Question. The electron distribution in an aluminium atom is
(a) 2, 8, 3
(b) 2, 8, 2
(c) 8, 2, 3
(d) 2, 3, 8
Answer. A
Question. In the Thomson’s model of atom, which of the following statements are correct?
(i) The mass of the atom is assumed to be uniformly distributed over the atom.
(ii) The positive charge is assumed to be uniformly distributed over the atom.
(iii) The electrons are uniformly distributed in the positively charged sphere.
(iv) The electrons attract each other to stabilise the atom.
(a) (i), (ii) and (iii)
(b) (i) and (iii)
(c) (i) and (iv)
(d) (i), (iii) and (iv)
Answer. A
Question. The relative atomic mass of naturally occurring chlorine is not a whole number. What is the reason for this?
(a) Chlorine atoms can have different numbers of neutrons.
(b) Naturally occurring chlorine cannot be obtained pure.
(c) Chlorine is unstable.
(d) The mass of the electrons has been included.
Answer. A
Question. Which one of the following is not isoelectronic with neon atom?
(a) 8O2–
(b) 11Na+
(c) 9F–
(d) 12Mg+
Answer. D
Question. The electronic configuration of elements
A, B, C and D are (2, 8, 1), (2, 8, 2), (2, 8, 6) and (2, 8, 7) respectively. Which of them can make an ion with two negative charges?
(a) A
(b) B
(c) C
(d) D
Answer. C
Question. Which pair of atoms contains the same number of neutrons?
(a) 11448Cd and 11950Sn
(b) 5927Co and 59 28Ni
(c) 13355Cs and 13254Xe
(d) 6329Cu and 6529Cu
Answer. C
Question. A has 9 protons, 9 electrons and 10 neutrons,B has 12 protons, 12 electrons and 12 neutrons.Formula of the compound between A and B is
(a) BA2
(b) AB2
(c) B2 A3
(d) AB4
Answer. A
Question. Match the column I with column II and select the correct answer by choosing an appropriate option.
Column I Column II
P. Mass of proton 1. 9.1 × 10–28 g
Q. Charge of electron 2. 1.6 × 10–19 C
R. Mass of electron 3. –1.6 × 10–19 C
S. Charge on proton 4. 1.67 × 10–27 kg
(a) P - 4, Q - 3, R - 1, S - 2
(b) P - 2, Q - 1, R - 4, S - 3
(c) P - 2, Q - 3, R - 4, S - 1
(d) P - 4, Q - 2, R - 1, S - 3
Answer. A
Question. Match the column I with column II and select the correct answer by choosing an appropriate option.
Column I Column II
P. Neutron 1. Rutherford’s atomic model
Q. Plum pudding model 2. Bohr’s atomic model
R. Mass of the atom is 3. Thomson’s atomic
concentrated at the model
centre of atom
S. Stationary orbit 4. Chadwick
(a) P - 1, 4; Q - 1, 2, 3; R - 2; S - 1, 2, 3
(b) P - 1; Q - 1, 2, 3; R - 2; S - 1, 2
(c) P - 1; Q - 3; R - 2; S - 1
(d) P - 4; Q - 3; R - 1; S - 2
Answer. D
Question. Match the following:-
List-I List-II
(P) Proton 1. Thomson
(Q) Electron 2. Goldstein
(R) Neutron 3. Rutherford
(S) Nucleus 4. Chadwick
(a) P-4, Q-3, R-2, S-1
(b) P-1, Q-2, R-3, S-4
(c) P-2, Q-1, R-4, S-3
(d) P-2, Q-1, R-3, S-4
Answer. C
Question. Match the following:-
List-I List-II
(P) Electrons 1. Number of positively charged particles in nucleus
(Q) Carbon dating 2. Negatively charged particles
(R) Valence electrons 3. Technique to know age of fossils
(S) Atomic number 4. Number of electrons in outermost shell
(a) P-1, Q-2, R-3, S-4
(b) P-4, Q-3, R-2, S-1
(c) P-2, Q-4, R-3, S-1
(d) P-2, Q-3, R-4, S-1
Answer. D
Case I : Read the passage given below and answer the following questions.
The maximum number of the electrons which are permitted to be assigned to an energy shell of an atom is called the electron capacity of that shell. The distribution of electrons in different orbits or shell is governed by a scheme known as Bohr-Bury scheme.
According to this scheme :
(i) The maximum number of the electrons that can be present in any shell is given by the formula 2n2 where, n is the number of energy level.
(ii) The maximum number of electrons that can be accommodated in the outermost shell is 8.
Electrons are filled in the shells in a stepwise manner in increasing order of energy of the energy shell.
Question. What is the maximum electrons capacity of N shell?
(a) 24
(b) 8
(c) 18
(d) 32
Answer. D
Question. Identify the element with the configuration K-2, L-8, M-3.
(a) Aluminium
(b) Magnesium
(c) Sodium
(d) Beryllium
Answer. A
Question. Which of the following configuration represent sodium?
(a) 2, 8, 4
(b) 2, 8, 5
(c) 2, 3
(d) 2, 8, 1
Answer. D
Case II : Read the passage given below and answer the following questions.
The table shows the number of sub-atomic particles in arbitrary elements, A to H.
Atom Number of protons Number of electrons Number of neutrons
A 1 1 0
B 3 3 4
C 4 4 6
D 5 5 5
E 6 6 6
F 6 6 7
G 9 9 10
H 9 9 11
Question. The pair of isotopes from the table is/are
(i) C and D
(ii) E and F
(iii) B and C
(iv) G and H
(a) (ii) only
(b) (iv) only
(c) (ii) and (iv) only
(d) (i), (ii), (iii) and (iv)
Answer. C
Question. Which of the given elements attains noble gas configuration by gaining an electron?
(i) A (ii) E
(iii) C (iv) H
(a) (iii) only
(b) (iv) only
(c) (i) and (iv) only
(d) (i) only
Answer. C
Question. Identify pair of isobars from the table.
(a) C and D
(b) B and E
(c) G and H
(d) E and F
Answer. A
Question. The atom _____ has nucleon number 13 and atom _____ has valency 3.
(a) G and F
(b) F and D
(c) C and E
(d) F and B
Answer. B
Case III : Look at the diagram given below and answer the following questions.
The given diagrams show the atomic structures of elements X and Y.
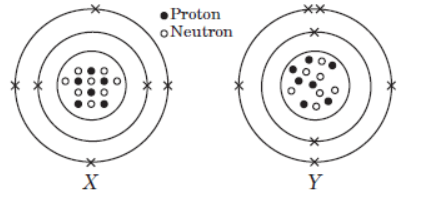
Question. Element X and Y could be _____ and _____respectively.
(a) Be and B
(b) C and O
(c) F and N
(d) C and N
Answer. D
Question.Valency of elements X and Y are respectively,
(a) 4 and 3
(b) 2 and 5
(c) 1 and 4
(d) 3 and 4
Answer. A
Question. Elements X and Y are
(a) isotopes
(b) isoelectronic
(c) isobars
(d) isomers.
Answer. C
Case IV : Read the passage given below and answer the following questions.
The mass of an atom is due to the masses of protons and neutrons in the nucleus. The relative masses of protons and neutrons are almost equal to one. Therefore, the atomic mass of an element should be nearly a whole number. But in many cases the atomic masses are fractional. The main reason for these fractional atomic masses is that these elements occur in nature as a mixture of several isotopes. The atomic mass of an element is the average of the atomic masses of these isotopes in the ratio of their proportion of occurrence.
Question. Chlorine occurs in nature in the form of two isotopes with atomic masses 35 u and 37 u in the ratio of 3 : 1 respectively. Atomic mass of chlorine is
(a) 35.5 u
(b) 34.5 u
(c) 35 u
(d) 36 u
Answer. A
Question. An element occurs in two isotopic forms with atomic masses 10 and 11. What is the percentage abundance of two isotopes in the sample having atomic mass 10.80?
(a) 20, 80
(b) 50, 50
(c) 25, 70
(d) 60, 40
Answer. A
Question. The fractional atomic masses of elements are due to the existence of
(a) isotopes having different masses
(b) diagonal relationship
(c) equal number of electrons and protons
(d) none of these.
Answer. A
Very Short Answer
Question. Who discovered electron?
Answer. J.J Thomson discovered electrons.
Question. What are canal rays?
Answer. Canal rays are positively charged radiations which led to the discovery of positively charged sub atomic particle called proton.
Question. What are the charged particles in matter?
Answer. When two objects rubbed together, the objects become electrically charged that is called charged particles of matter.
Question. Who is the father of nuclear physics?
Answer. E. Rutherford was known as the Father of the nuclear Physics.
Question. Who and when discovered canal rays?
Answer. In 1886 E. Goldstein discovered canal rays.
Short Answer
Question. What is sub atomic particle?
Answer. Subatomic particle also known as elementary particle. Subatomic particles include electrons, the negatively charged, almost massless particles that nevertheless account for most of the size of the atom, and they include the heavier building blocks of the small but very dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.
Question. What is the structure of an atom?
Answer. Atoms consist of three basic particles: protons, electrons, and neutrons.The nucleus (centre) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
Question. What happens when we combs dry hair? Does the comb then attract small pieces of paper?
Answer. When the comb is rubbed against the cloth the electrons move between the two, and the comb gets charged. The same thing happens when the comb is brought near neutral paper strips. Within the papers strips, the electrons get either repelled or attracted according to whether the comb has a negative or a positive charge.
Question. Helium atom has an atomic mass of 4 u and two protons in its nucleus? How many neutrons does it have?
Answer. The mass of an atom is given by the sum of the masses of protons and neutrons present in the nucleus. Since helium atom has an atomic mass of 4 u and it has two protons. Two protons contribute 2 u to atomic mass. Hence, it must contain 2 neutrons.
Question. Name the three sub atomic particles of an atom?
Answer. The three main subatomic particles that form an atom are protons, neutrons,and electrons. The centre of the atom is called the nucleus. Protons and neutrons are located in the nucleus, a dense central core in the middle of the atom, while the electrons are located outside the nucleus.
Long Answer
Question. Explain the Thomson’s model of an Atom?
Answer. Thomson atomic model was proposed by William Thomson in the year 1900. It was strongly supported by J.J Thomson, who had discovered the electron. Thomson's model,the atom is composed of electrons surrounded by a soup of positive charge to balance the electrons' negative charges, like negatively charged “plums” surrounded by positively charged “pudding”. Thomson assumed that an electron is two thousand times lighter than a proton and believed that an atom is made up of thousands of electrons. In this atomic structure model, he considered atoms surrounded by a cloud having positive as well as negative charges. Thomson’s model of an atom is similar to a plum pudding. Thomson proposed that:
1. An atom consists of a positively charged sphere and the electrons are embedded in it.
2. The negative and the positive charges are equal in magnitude. So the atom as a whole is electrically neutral.
Question. What happens 1. When we rub aglass rod with a silk cloth and bring the rod near an inflated balloon? 2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer. Answer for 1st part:
The core has positive charge, the electrons have negative charge. When you are rubbing the glass rod with the silk cloth, electrons are stripped away from the atoms in the glass and transferred to the silk cloth. This leaves the glass rod with more positive than negative charge, so we get a positive charge. When we take it near the balloon, the
balloon will get attracted.as while rubbing the glass rod with silk cloth the rod gets charged and the charged object attract an uncharged object, so they will show the attraction.
Answer for 2nd part:
An electron is a negatively charged particle, whereas a proton is a positively charged particle. The magnitude of their charge is equal. Therefore, an atom containing one electron and one proton will not carry any charge. So, it will be a neutral atom
Question. What is the Rutherford’s model of an Atom?
Answer. Rutherford’s conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil.Rutherford, in his experiment, directed high energy streams of α-particles from a radioactive source at a thin sheet (100 nm thickness) of gold. In order to study the deflection caused to the α-particles, he placed a fluorescent zinc sulphide screen around the thin gold foil.
1. He selected a gold foil because he wanted as thin a layer as possible. This gold
foil was about 1000 atoms thick.
2. α- particles are doubly charged helium ions, since they have a mass of 4 u the fast moving α- particles have a considerable amount of energy.
3. It was expected that α- particles would be deflected by the sub atomic particles in the gold atoms. So the α-particles were much heavier than the protons.
But the α-particle scattering experiment gave the unexpected results:
1. Most of the fast moving α-particles passed straight through the gold foil.
2. Some of the α-particles were deflected by the foil by small angles.
3. Surprisingly, one out of the every 12000 particles appeared to rebound.
Question. What are the drawbacks of Rutherford’s model of the atom?
Answer. Rutherford atomic model was the first step in the evolution of the modern atomic model.
Ernest Rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. He performed an experiment using alpha particles and gold foil and made the following drawbacks:
1. Rutherford atomic model could not explain how the moving electrons could remain in its orbit.
2. Most of the α- particles passed straight through gold foil.
3. There was a deflection of the α- particles by a small angle.
4. Any charged particle during acceleration would give out energy and while revolving it would lose energy and eventually fall into nucleus.
5. The atom should be highly unstable.
6. Matter is composed of stable form of atoms.
7. Its major drawback was that it could not explain the stability of atoms.
Question. Explain the structure of an atom?
Answer. Structure of an atom can be basically divided into two parts: an atomic nucleus, extra nucleus part. The tiny atomic nucleus is the centre of an atom constituting positively charged particles “protons” and uncharged particles “neutrons.” The extra nucleus part is a much larger region which is composed of a cloud of negatively charged particles called an electron. Electrons revolve around the orbit or centre of the nucleus. The attraction between the protons and electrons holds the structure of an atom together. Atoms are made of three different kinds of subatomic particles: neutrons, protons and electrons. The nucleus, or centre of an atom, is made of protons, which are positively charged particles and neutrons, which are neutral (have no charge). Electrons are negatively charged particles. The atomic nucleus in the structure of the atom is composed of a fixed number of protons and the proton attracts the same number of electrons thereby making an atom electrically neutral. Ions are formed by addition or removal of electrons from an atom.
Click below to download practice worksheet for CBSE Class 9 Science Structure of The Atom Worksheet Set A
| CBSE Class 9 Science Work and Energy Worksheet Set A |
| CBSE Class 9 Science Work and Energy Worksheet Set B |
| CBSE Class 9 Science Sound Worksheet Set A |
| CBSE Class 9 Science Sound Worksheet Set B |
| CBSE Class 9 Science Improvent in Food Resources Worksheet Set A |
| CBSE Class 9 Science Improvent in Food Resources Worksheet Set B |
| CBSE Class 9 Science Why Do We Fall ill Worksheet Set A |
| CBSE Class 9 Science Why Do We Fall ill Worksheet Set B |
Chapter 4 Structure of the Atom CBSE Class 9 Science Worksheet
The above practice worksheet for Chapter 4 Structure of the Atom has been designed as per the current syllabus for Class 9 Science released by CBSE. Students studying in Class 9 can easily download in Pdf format and practice the questions and answers given in the above practice worksheet for Class 9 Science on a daily basis. All the latest practice worksheets with solutions have been developed for Science by referring to the most important and regularly asked topics that the students should learn and practice to get better scores in their examinations. Studiestoday is the best portal for Printable Worksheets for Class 9 Science students to get all the latest study material free of cost. Teachers of studiestoday have referred to the NCERT book for Class 9 Science to develop the Science Class 9 worksheet. After solving the questions given in the practice sheet which have been developed as per the latest course books also refer to the NCERT solutions for Class 9 Science designed by our teachers. After solving these you should also refer to Class 9 Science MCQ Test for the same chapter. We have also provided a lot of other Worksheets for Class 9 Science which you can use to further make yourself better in Science.
You can download the CBSE Practice worksheets for Class 9 Science Chapter 4 Structure of the Atom for the latest session from StudiesToday.com
Yes, the Practice worksheets issued for Chapter 4 Structure of the Atom Class 9 Science have been made available here for the latest academic session
There is no charge for the Practice worksheets for Class 9 CBSE Science Chapter 4 Structure of the Atom you can download everything free
Regular revision of practice worksheets given on studiestoday for Class 9 subject Science Chapter 4 Structure of the Atom can help you to score better marks in exams
Yes, studiestoday.com provides all the latest Class 9 Science Chapter 4 Structure of the Atom test practice sheets with answers based on the latest books for the current academic session

