NCERT Solutions Class 9 Science Chapter 4 Structure of the Atom have been provided below and is also available in Pdf for free download. The NCERT solutions for Class 9 Science have been prepared as per the latest syllabus, NCERT books and examination pattern suggested in Class 9 by CBSE, NCERT and KVS. Questions given in NCERT book for Class 9 Science are an important part of exams for Class 9 Science and if answered properly can help you to get higher marks. Refer to more Chapter-wise answers for NCERT Class 9 Science and also download more latest study material for all subjects. Chapter 4 Structure of the Atom is an important topic in Class 9, please refer to answers provided below to help you score better in exams
Chapter 4 Structure of the Atom Class 9 Science NCERT Solutions
Class 9 Science students should refer to the following NCERT questions with answers for Chapter 4 Structure of the Atom in Class 9. These NCERT Solutions with answers for Class 9 Science will come in exams and help you to score good marks
Chapter 4 Structure of the Atom NCERT Solutions Class 9 Science
Class IX Science
Chapter 4 – Structure of the Atom
Question 1: What are canal rays?
Answer : Canal ra ys are positively charged radiations. These rays consist of positively charged particles known as protons. They were discovered by Goldstein in 1886.
Question 2: If an atom contains one electron and one proton, will it carry any charge or not?
Answer : An electron is a negatively charged particle, whereas a proton is a positively charged particle. The magnitude of their charges is equal. Therefore, an atom containing one electron and one proton will not carry any charge. Thus, it will be a neutral atom.
Chapter 4 – Structure of the Atom
Question 1: On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer : According to Thomson’s model of the atom, an atom consists of both negatively and positively charged particles. The negatively charged particles are embedded in the positively charged sphere. These negative and positive charges are equal in magnitude. Thus, by counterbalancing each other’s effect, they make an atom neutral.
Question 2: On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer : On the basis of Rutherford's model of an atom, protons (positively-charged particles) are present in the nucleus of an atom.
Question 3: Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
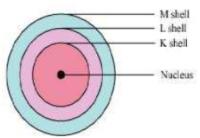
Bohr’s model of an atom with three shells
Question 4: What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
Answer: If the α-scattering experiment is carried out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold
foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from other metals.
Chapter 4 – Structure of the Atom
Question 1: Name the three sub-atomic particles of an atom.
Answer: The three sub-atomic particles of an atom are:
(i) Protons
(ii) Electrons, and
(iii) Neutrons
Question 2: Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer: Helium atom has two neutrons. The mass of an atom is the sum of the masses of protons and neutrons present in its nucleus. Since helium atom has two protons, mass contributed by the two protons is (2 × 1) u = 2 u. Then, the remaining mass
(4 − 2) u = 2 u is contributed by 2u/1u = 2 neutrons.
Chapter 4 – Structure of the Atom
Question 1: Write the distribution of electrons in carbon and sodium atoms?
Answer: Thetotal number of electrons in a carbon atom is 6. The distribution of electrons in carbon atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 4 electrons
Or, we can write the distribution of electrons in a carbon atom as 2, 4.
The total number of electrons in a sodium atom is 11. The distribution of electrons in sodium atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 8 electrons
Third orbit or M-shell = 1 electron
Or, we can write distribution of electrons in a sodium atom as 2, 8, 1.
Question 2: If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer: The maximum number of electrons that can occupy K and L-shells of an atom are 2 and 8 respectively. Therefore, if K and L-shells of an atom are full, then the total number of electrons in the atom would be (2 + 8) = 10 electrons.
Chapter 4 – Structure of the Atom
Question 1: How will you find the valency of chlorine, sulphur and magnesium?
Answer: If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell. On the other hand, if the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
The distribution of electrons in chlorine, sulphur, and magnesium atoms are 2, 8, 7; 2, 8, 6 and 2, 8, 2 respectively.
Therefore, the number of electrons in the outer most shell of chlorine, sulphur, and magnesium atoms are 7, 6, and 2 respectively.
Thus, the valency of chlorine = 8 −7 = 1
The valency of sulphur = 8 − 6 = 2
The valency of magnesium = 2
Chapter 4 – Structure of the Atom
Question 1: If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom and (ii) what is the charge on the atom?
Answer:
(i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8.
(ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0.
Question 2: With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Answer:
Mass number of oxygen = Number of protons + Number of neutrons
= 8 + 8
= 16
Mass number of sulphur = Number of protons + Number of neutrons
= 16 +16
= 32
Chapter 4 – Structure of the Atom
Question 1: For the symbol H, D and T tabulate three sub-atomic particles found in each of them.
Answer:
| Symbol | Proton | Neutron | Electron |
| H | 1 | 0 | 1 |
| D | 1 | 1 | 1 |
| T | 1 | 2 | 1 |
Question 2: Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
Two isotopes of carbon are 126C and 146C .
The electronic configuration of 126C is 2, 4.
The electronic configuration of 146C is 2, 4.
[Isotopes have the same electronic configuration]
4020Ca and 4018Ar are a pair of isobars
The electronic configuration of 4020Ca is 2, 8, 8, 2.
The electronic configuration of 4018Ar is 2, 8, 8.
Chapter 4 – Structure of the Atom
Question 1: Compare the properties of electrons, protons and neutrons.
Answer:
| Electron | Proton | Neutron | |||
| (i) | Electrons are | (i) | Protons are present in the | (i) | Neutrons are |
| present outside | nucleus of an atom. | present in the | |||
| the nucleus of an | nucleus of an | ||||
| atom. | atom. | ||||
| (ii) | Electrons are | (ii) | Protons are positively | (ii) | Neutrons are |
| negatively | charged. | neutral. | |||
| charged. | |||||
| (iii) | The mass of an | (iii) | The mass of a proton is | (iii) | The mass of |
| electron is | approximately 2000 | neutron is nearly | |||
| considered to | times as the mass of an | equal to the mass | |||
| negligible. | electron. | of a proton. |
Question 2: What are the limitations of J.J. Thomson’s model of the atom?
Answer:According to J.J. Thomson’s model of an atom, an atom consists of a positively charged sphere with electrons embedded in it. However, it was later found that the positively charged particles reside at the centre of the atom called the nucleus, and the electrons revolve around the nucleus.
Question 3: What are the limitations of Rutherford’s model of the atom?
Answer: According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed orbits. But, an electron revolving in circular orbits will not be stable because during revolution, it will experience acceleration. Due to acceleration, the electrons will lose energy in the form of radiation and fall into the nucleus. In such a case, the atom would be highly unstable and collapse.
Question 4: Describe Bohr’s model of the atom.
Answer: Bohr’s model of the atom
Niels Bohr proposed the following postulates regarding the model of the atom.
(i) Only certain orbits known as discrete orbits of electrons are allowed inside the atom.
(ii) While revolving in these discrete orbits, the electrons do not radiate energy.
These discrete orbits or shells are shown in the following diagram.

The first orbit (i.e., for n = 1) is represented by letter K. Similarly, for n = 2, it is L − shell, for n = 3, it is M − shell and for n = 4, it is N − shell. These orbits or shells are also called energy levels.
Question 5: Compare all the proposed models of an atom given in this chapter.
Answer :
| Thomson’s Model | Rutherford’s model | Bohr’s model |
| An atom consists | An atom consists of a positively | There are only certain |
| of a positively | charged particles concentrated at | orbits known as discrete |
| charged sphere | the centre known as the nucleus. | orbits inside the atom in |
| with electrons | The size of the nucleus is very small | which electrons revolve |
| embedded in it. | as compared to the size of the | around the nucleus. |
| atom. The electrons revolve around | Electrons do not radiate | |
| the nucleus in well-defined orbits. | energy while revolving. |
Question 6: Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer: The rules for writing of the distribution of electrons in various shells for the first eighteen elements are given below.
(i) The maximum number of electrons that a shell can accommodate is given by the formula ‘2n2’, where ‘n’ is the orbit number or energy level index (n = 1, 2, 3…).
The maximum number of electrons present in an orbit of n = 1 is given by 2n2 = 2×12 = 2
Similarly, for second orbit, it is 2n2 = 2×22 = 8
For third orbit, it is 2n2 = 2×32 = 18
And so on……
(ii) The outermost orbit can be accommodated by a maximum number of 8 electrons.
(iii) Shells are filled with electrons in a stepwise manner i.e., the outer shell is not occupied with electrons unless the inner shells are completely filled with electrons.
Question 7: Define valency by taking examples of silicon and oxygen.
Answer: The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element. If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons. Thus, the valency of silicon is four. On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight. For example, the atom of oxygen has six valence electrons. Thus, the valency of oxygen is (8 − 6) i.e., two.
Question 8: Explain with examples
(i) Atomic number,
(ii) Mass number,
(iii) Isotopes and
(iv) Isobars. Give any two uses of isotopes.
Answer: (i) Atomic number The atomic number of an element is the total number of protons present in the atom of that element. For example, nitrogen has 7 protons in its atom. Thus, the atomic number of nitrogen is 7.
(ii) Mass number The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11.
(iii) Isotopes Isotopes are atoms of the same element having the same atomic number, but different mass numbers. For example, hydrogen has three isotopes. They are protium (11H) , deuterium , (21H) and tritium (31H)
(iv) Isobars Isobars are atoms having the same mass number, but different atomic numbers i.e., isobars are atoms of different elements having the same mass number. For example, 4020Ca and 4018Ar are isobars.
Two uses of isotopes are:
(i) One isotope of uranium is used as a fuel in nuclear reactors.
(ii) One isotope of cobalt is used in the treatment of cancer.
Question 9: Na+ has completely filled K and L shells. Explain.
Answer: An atom of Na has a total of 11 electrons. Its electronic configuration is 2, 8, 1. But, Na+ ion has one electron less than Na atom i.e., it has 10 electrons. Therefore, 2
electrons go to K-shell and 8 electrons go to L-shell, thereby completely filling K and L shells.
Question 10: If bromine atom is available in the form of, say, two isotopes 7935Br (49.7%) and 8135Br (50.3%) calculate the average atomic mass of bromine atom.
Answer: It is given that two isotopes of bromine are 7935Br (49.7%) and 8135Br (50.3%) Then, the average atomic mass of bromine atom is given by:
79 x 49.7 /100 + 81 x 50.3 /100
= 3926.3 /100 + 4074.3 /100
= 8000.6/100
= 80.006 u
= 80 u (approx)
Question 11: The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 188X in the sample?
Answer: It is given that the average atomic mass of the sample of element X is 16.2 u.
Let the percentage of isotope 188X be y%. Thus, the percentage of isotope 168X will be (100 − y) %.
Therefore,
Therefore, the percentage of isotope 188X is 10%.
And, the percentage of isotope 168X is (100 − 10) % = 90%.
Question 12: If Z = 3, what would be the valency of the element? Also, name the element.
Answer: By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium.
Question 13: Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Answer: Mass number of X = Number of protons + Number of neutrons
= 6 + 6
= 12
Mass number of Y = Number of protons + Number of neutrons
= 6 + 8
= 14
These two atomic species X and Y have the same atomic number, but different mass numbers. Hence, they are isotopes.
Question 14: For the following statements, write T for ‘True’ and F for ‘False’.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (F)
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (F)
(c) The mass of an electron is about 1/2000 times that of proton. (T)
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. (T)
Question 15: Put tick (✓) against correct choice and cross (X) against wrong choice in the following question:
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus
(b) Electron
(c) Proton
(d) Neutron
Answer: Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus (✓)
(b) Electron (X)
(c) Proton (X)
(d) Neutron (X)
Put tick (✓) against correct choice and cross (X) against wrong choice in the following question:
Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
Answer: Isotopes of an element have
(a) the same physical properties (X)
(b) different chemical properties (X)
(c) different number of neutrons (✓)
(d) different atomic numbers (X)
Question 17: Put tick (✓) against correct choice and cross (X) against wrong choice in the following question:
Number of valence electrons in Cl− ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer: Number of valence electrons in Cl− ion are:
(a) 16 (X)
(b) 8 (✓)
(c) 17 (X)
(d) 18 (X)
Question 18: Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
Answer: (d) The correct electronic configuration of sodium is 2, 8, 1.
Question 19: Complete the following table.
| Atomic number | Mass number | Number of Neutrons | Number of protons | Number of electrons | Name of the Atomic species |
| 9 | − | 10 | − | − | − |
| 16 | 32 | − | − | − | Sulphur |
| − | 24 | − | 12 | − | − |
| − | 2 | − | 1 | − | − |
| − | 1 | 0 | 1 | 1 | − |
Answer :
| Atomic number | Mass number | Number of Neutrons | Number of protons | Number of electrons | Name of the Atomic species |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulphur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 0 | 1 | 1 | Protium |
Question. Atoms consist of electrons, protons and neutrons. Isotopes of an element show similar chemical properties, but have different atomic weights. Thus they are likely to have:
(a) same number of electrons, protons and neutrons.
(b) same number of electrons and neutrons; different number of protons
(c) same number of neutrons and protons; different number of electrons
(d) same number of electrons and protons; different number of neutrons
Answer : D
Question. We know that like charges repel each other. Then how do the protons, which are all positively charged, stay together in an atom's nucleus?
(a) The neutral charge of the neutron keeps them together.
(b) Nuclei keep decaying in short intervals because of this.
(c) The nucleic force is stronger than their mutual repulsion
(d) That like charges repel is not true at the level of the nucleus.
Answer : C
Question. The number of protons and neutrons in the atom of an element are represented in the given form.Uranium-238 undergoes radioactive decay by losing an alpha particle to produce Thorium. This is expressed mathematically by the following equation.What is the number of protons and neutrons in an alpha particle?
(a) 2 protons and no neutrons
(b) 4 protons and no neutrons
(c) 2 protons and 2 neutrons
(d) No protons and 4 neutrons
Answer : C
Question. The half-life of a radioactive material is the time in which half its atoms decay. Technetium-99 is a radioactive material with a half-life of 6 days. It is used to study blood flow around the body. A sample of technetium-99 has an activity of 96 counts per minute when injected into a patient’s blood stream. Its activity after 18 days would be
(a) 48 counts per minute
(b) 24 counts per minute
(c) 16 counts per minute
(d) 12 counts per minute
Answer : D
Question. Compared to a chlorine atom, Cl, a chlorine ion Cl-, will have _______
(a) one proton less
(b) one neutron more
(c) one electron more
(d) one neutron less and one proton less
Answer : C
Question. Which element is found in both sodium chlorate and zinc nitrate?
(a) nitrogen
(b) Hydrogen
(c) oxygen
(d) Chlorine
Answer : C
| NCERT Solutions Class 9 Science Chapter 1 Matter in Our Surroundings |
| NCERT Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure |
| NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules |
| NCERT Solutions Class 9 Science Chapter 4 Structure of the Atom |
| NCERT Solutions Class 9 Science Chapter 5 The Fundamental Unit of Life |
| NCERT Solutions Class 9 Science Chapter 6 Tissues |
| NCERT Solutions Class 9 Science Chapter 7 Diversity in Living Organisms |
| NCERT Solutions Class 9 Science Chapter 8 Motion |
| NCERT Solutions Class 9 Science Chapter 9 Force and Laws of Motion |
| NCERT Solutions Class 9 Science Chapter 10 Gravitation |
| NCERT Solutions Class 9 Science Chapter 11 Work and Energy |
| NCERT Solutions Class 9 Science Chapter 12 Sound |
| NCERT Solutions Class 9 Science Chapter 13 Why Do We Fall Ill |
| NCERT Solutions Class 9 Science Chapter 14 Natural Resources |
| NCERT Solutions Class 9 Science Chapter 15 Improvement in Food Resources |
NCERT Solutions Class 9 Science Chapter 4 Structure of the Atom
The above provided NCERT Solutions Class 9 Science Chapter 4 Structure of the Atom is available on our website www.studiestoday.com for free download in Pdf. You can read the solutions to all questions given in your Class 9 Science textbook online or you can easily download them in pdf. The answers to each question in Chapter 4 Structure of the Atom of Science Class 9 has been designed based on the latest syllabus released for the current year. We have also provided detailed explanations for all difficult topics in Chapter 4 Structure of the Atom Class 9 chapter of Science so that it can be easier for students to understand all answers. These solutions of Chapter 4 Structure of the Atom NCERT Questions given in your textbook for Class 9 Science have been designed to help students understand the difficult topics of Science in an easy manner. These will also help to build a strong foundation in the Science. There is a combination of theoretical and practical questions relating to all chapters in Science to check the overall learning of the students of Class 9.
You can download the NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom for latest session from StudiesToday.com
Yes, the NCERT Solutions issued for Class 9 Science Chapter 4 Structure of the Atom have been made available here for latest academic session
Regular revision of NCERT Solutions given on studiestoday for Class 9 subject Science Chapter 4 Structure of the Atom can help you to score better marks in exams
Yes, studiestoday.com provides all latest NCERT Chapter 4 Structure of the Atom Class 9 Science solutions based on the latest books for the current academic session
Yes, NCERT solutions for Class 9 Chapter 4 Structure of the Atom Science are available in multiple languages, including English, Hindi
All questions given in the end of the chapter Chapter 4 Structure of the Atom have been answered by our teachers

