NCERT Solutions Class 10 Science Chapter 5 Periodic Classification of Elements have been provided below and is also available in Pdf for free download. The NCERT solutions for Class 10 Science have been prepared as per the latest syllabus, NCERT books and examination pattern suggested in Class 10 by CBSE, NCERT and KVS. Questions given in NCERT book for Class 10 Science are an important part of exams for Class 10 Science and if answered properly can help you to get higher marks. Refer to more Chapter-wise answers for NCERT Class 10 Science and also download more latest study material for all subjects. Chapter 5 Periodic Classification of Elements is an important topic in Class 10, please refer to answers provided below to help you score better in exams
Chapter 5 Periodic Classification of Elements Class 10 Science NCERT Solutions
Class 10 Science students should refer to the following NCERT questions with answers for Chapter 5 Periodic Classification of Elements in Class 10. These NCERT Solutions with answers for Class 10 Science will come in exams and help you to score good marks
Chapter 5 Periodic Classification of Elements NCERT Solutions Class 10 Science
Question : Did Dobereiner’s triads also exist in the columns of Newlands’ Octaves? Compare and find out.
Answer: Only one triad of Dobereiner’s triads exists in the columns of Newlands’ octaves. The triad formed by the elements Li, Na, and K of Dobereiner’s triads also occurred in the columns of Newlands’ octaves.
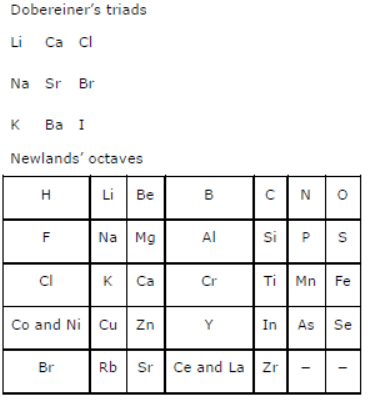
Question : What were the limitations of Dobereiner’s classification?
Answer: Limitation of Dobereiner’s classification: All known elements could not be classified into groups of triads on the basis of their properties.
Question : What were the limitations of Newlands’ Law of Octaves?
Answer: Limitations of Newlands’ law of octaves:
(i) It was not applicable throughout the arrangements. It was applicable up to calcium only. The properties of the elements listed after calcium showed no resemblance to the properties of the elements above them.
(ii) Those elements that were discovered after Newlands’ octaves did not follow the law of octaves.
(iii) The position of cobalt and nickel in the group of the elements (F, Cl) of different properties could not be explained.
(iv) Placing of iron far away from cobalt and nickel, which have similar properties as iron, could also not be explained.
Question : Use Mendeleev’s Periodic Table to predict the formulae for the oxides of the following elements: K, C, Al, Si, Ba.
Answer:
K is in group 1. Therefore, the oxide will be K2O.
C is in group 4. Therefore, the oxide will be CO2.
Al is in group 3. Therefore, the oxide will be Al2O3.
Si is in group 4. Therefore, the oxide will be SiO2.
Ba is in group 2. Therefore, the oxide will be BaO.
Question : Besides gallium, which other elements have since been discovered that were left by Mendeleev in his Periodic Table? (any two)
Answer: Scandium and germanium
Question : What were the criteria used by Mendeleev in creating his Periodic Table?
Answer: Mendeleev’s periodic table was based on the observation that the properties of elements are a periodic function of their atomic masses. This means that if elements are arranged in the increasing order of their atomic masses, then their properties get repeated after regular intervals.
Question : Why do you think the noble gases are placed in a separate group?
Answer: Noble gases are inert elements. Their properties are different from the all other elements. Therefore, the noble gases are placed in a separate group.
Question : How could the Modern Periodic Table remove various anomalies of Mendeleev’s Periodic Table?
Answer: Mendeleev was unable to give fixed position to hydrogen and isotopes in the periodic table. In Mendeleev’s periodic table, the increasing manner of atomic mass of the elements is not always regular from one to its next. It was believed that a more fundamental property than atomic mass could explain periodic properties in a better manner.
It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an element and arranged the elements in increasing order of their atomic numbers. Then it was found that the various anomalies of Mendeleev’s periodic table were removed by the modern periodic table.
Question : Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice?
Answer: Calcium (Ca) and strontium (Sr) are expected to show chemical reactions similar tomagnesium (Mg). This is because the number of valence electrons (2) is same in all these three elements. And since chemical properties are due to valence electrons, they show same chemical reactions.
Question : Name
(a) three elements that have a single electron in their outermost shells.
(b) two elements that have two electrons in their outermost shells.
(c) three elements with filled outermost shells.
Answer: (a) Lithium (Li), sodium (Na), and potassium (K) have a single electron in their outermost shells.
(b) Magnesium (Mg) and calcium (Ca) have two electrons in their outermost shells.
(c) Neon (Ne), argon (Ar), and xenon (Xe) have filled outermost shells.
Question : (a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements?
(b) Helium is an unreactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common?
Answer:
(a) Yes. The atoms of all the three elements lithium, sodium, and potassium have one electron in their outermost shells.
(b) Both helium (He) and neon (Ne) have filled outermost shells. Helium has a duplet in its K shell, while neon has an octet in its L shell.
Question : In the Modern Periodic Table, which are the metals among the first ten elements?
Answer: Among the first ten elements, lithium (Li) and beryllium (Be) are metals.
Question : By considering their position in the Periodic Table, which one of the following elements would you expect to have maximum metallic characteristic?
Answer: Since Be lies to the extreme left hand side of the periodic table, Be is the most metallic among the given elements.
Question : Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic Table.
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
Answer:
(c) The atoms lose their electrons more easily.
(On moving from left to right across the periods of the periodic table, the non-metallic character increases. Hence, the tendency to lose electrons decreases.)
Question : Element X forms a chloride with the formula XCl2, which is a solid with a high melting point. X would most likely be in the same group of the Periodic Table as
(a) Na (b) Mg (c) Al (d) Si
Answer:
(b) X would most likely be in the same group of the Periodic Table as magnesium (Mg).
Question : Which element has
(a) two shells, both of which are completely filled with electrons?
(b) the electronic configuration 2, 8, 2?
(c) a total of three shells, with four electrons in its valence shell?
(d) a total of two shells, with three electrons in its valence shell?
(e) twice as many electrons in its second shell as in its first shell?
Answer:
(a) Neon has two shells, both of which are completely filled with electrons (2 electrons in K shell and 8 electrons in L shell).
(b) Magnesium has the electronic configuration 2, 8, 2.
(c) Silicon has a total of three shells, with four electrons in its valence shell (2 electrons in K shell, 8 electrons in L shell and 4 electrons in M shell).
(d) Boron has a total of two shells, with three electrons in its valence shell (2 electrons in K shell and 3 electrons in L shell)
(e) Carbon has twice as many electrons in its second shell as in its first shell (2 electrons in K shell and 4 electrons in L shell).
Question :
(a) What property do all elements in the same column of the Periodic Table as boron have in common?
(b) What property do all elements in the same column of the Periodic Table as fluorine have in common?
Answer:
(a) All the elements in the same column as boron have the same number of valence electrons (3). Hence, they all have valency equal to 3.
(b) All the elements in the same column as fluorine have the same number of valence electrons (7). Hence, they all have valency equal to 1.
Question : An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element?
(b) To which of the following elements would it be chemically similar? (Atomic numbers are given in parentheses.)
N(7) F(9) P(15) Ar(18)
Answer:
(a) The atomic number of this element is 17.
(b) It would be chemically similar to F(9) with configuration as 2, 7.
Question :
The position of three elements A, B and C in the Periodic Table are shown below −
Group 16 Group 17
− −
− A
− −
B C
(a) State whether A is a metal or non-metal.
(b) State whether C is more reactive or less reactive than A.
(c) Will C be larger or smaller in size than B?
(d) Which type of ion, cation or anion, will be formed by element A?
Answer:
(a) A is a non-metal.
(b) C is less reactive than A, as reactivity decreases down the group in halogens.
(c) C will be smaller in size than B as moving across a period, the nuclear charge increases and therefore, electrons come closer to the nucleus.
(d) A will form an anion as it accepts an electron to complete its octet.
Question : Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Answer:
Element K L M
Nitrogen 2 5
Phosphorus 2 8 5
Nitrogen is more electronegative than phosphorus. On moving down a group, the number of shell increases. Therefore, the valence electrons move away from the nucleus and the effective nuclear charge decreases. This causes the decrease in the tendency to attract electron and hence electronegativity decreases.
Question : How does the electronic configuration of an atom relate to its position in the Modern Periodic Table?
Answer: In the modern periodic table, atoms with similar electronic configurations are placed in the same column. In a group, the number of valence electrons remains the same.Elements across a period show an increase in the number of valence electrons.
Question : In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21, and 38. Which of these have physical and chemical properties resembling calcium?
Answer: The element with atomic number 12 has same chemical properties as that of calcium.
This is because both of them have same number of valence electrons (2).
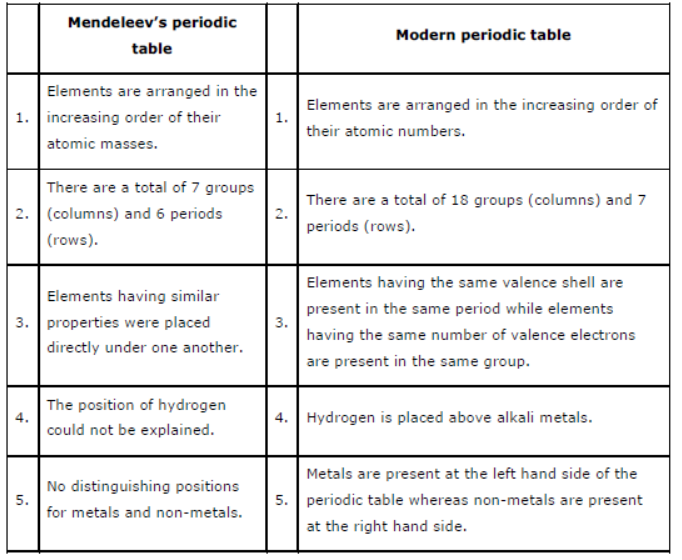
Question : Compare and contrast the arrangement of elements in Mendeleev’s periodic Table and the Modern Periodic Table.
Answer:
| NCERT Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations |
| NCERT Solutions Class 10 Science Chapter 2 Acids Bases and Salts |
| NCERT Solutions Class 10 Science Chapter 3 Metals and Non metals |
| NCERT Solutions Class 10 Science Chapter 4 Carbon and its Compounds |
| NCERT Solutions Class 10 Science Chapter 5 Periodic Classification of Elements |
| NCERT Solutions Class 10 Science Chapter 6 Life Processes |
| NCERT Solutions Class 10 Science Chapter 7 Control and Coordination |
| NCERT Solutions Class 10 Science Chapter 8 How do Organisms Reproduce |
| NCERT Solutions Class 10 Science Chapter 9 Heredity and Evolution |
| NCERT Solutions Class 10 Science Chapter 10 Light Reflection and Refraction |
| NCERT Solutions Class 10 Science Chapter 11 Human Eye and Colourful World |
| NCERT Solutions Class 10 Science Chapter 12 Electricity |
| NCERT Solutions Class 10 Science Chapter 13 Magnetic Effects of Electric current |
| NCERT Solutions Class 10 Science Chapter 14 Sources of Energy |
| NCERT Solutions Class 10 Science Chapter 15 Our Environment |
| NCERT Solutions Class 10 Science Chapter 16 Management of Natural Resources |
NCERT Solutions Class 10 Science Chapter 5 Periodic Classification of Elements
The above provided NCERT Solutions Class 10 Science Chapter 5 Periodic Classification of Elements is available on our website www.studiestoday.com for free download in Pdf. You can read the solutions to all questions given in your Class 10 Science textbook online or you can easily download them in pdf. The answers to each question in Chapter 5 Periodic Classification of Elements of Science Class 10 has been designed based on the latest syllabus released for the current year. We have also provided detailed explanations for all difficult topics in Chapter 5 Periodic Classification of Elements Class 10 chapter of Science so that it can be easier for students to understand all answers. These solutions of Chapter 5 Periodic Classification of Elements NCERT Questions given in your textbook for Class 10 Science have been designed to help students understand the difficult topics of Science in an easy manner. These will also help to build a strong foundation in the Science. There is a combination of theoretical and practical questions relating to all chapters in Science to check the overall learning of the students of Class 10.
You can download the NCERT Solutions for Class 10 Science Chapter 5 Periodic Classification of Elements for latest session from StudiesToday.com
Yes, the NCERT Solutions issued for Class 10 Science Chapter 5 Periodic Classification of Elements have been made available here for latest academic session
Regular revision of NCERT Solutions given on studiestoday for Class 10 subject Science Chapter 5 Periodic Classification of Elements can help you to score better marks in exams
Yes, studiestoday.com provides all latest NCERT Chapter 5 Periodic Classification of Elements Class 10 Science solutions based on the latest books for the current academic session
Yes, NCERT solutions for Class 10 Chapter 5 Periodic Classification of Elements Science are available in multiple languages, including English, Hindi
All questions given in the end of the chapter Chapter 5 Periodic Classification of Elements have been answered by our teachers

