CHAPTER:4, CHEMICAL KINETICS
1 - MARK QUESTIONS
Question. The half life period of two samples are 0.1 and 0.4 seconds. Their initial concentrations are 200 and 50 mol L -1 respectively. What is the order of reaction?
Answer: When the initial conc. become ¼, half life becomes 4 times. Order of reaction is 2.
Question. What is the ratio of t3/4 : t1/2 for a first order reaction ?
Answer: 2:1
Question. Higher molecularity reactions (viz. molecularity, 4 and above) are very rare. Why?
Answer: Molecules of reactants collide simultaneously and go to products. The probability of colliding 4 or 5 species simultaneously is very rare.
Question. Consider the reaction 2A + B à Products. When concentration of B alone was doubled, half life time does not change. When conc. of A alone doubled, the rate increases by two times . What are the units of K and what is the order of reaction?
Answer: The half life is independent of conc. of B. This means the reaction is of first order w.r.t. B and w.r.t. A also it is first order and overall order is second.
Question. How is half life time related to initial conc. for a second order reaction?
Answer: t1/2 ∞(conc.)1-n n=2
t1/2 µ(conc.)-1 or
Question. The rate of reaction is given by K = P.Z.e-Ea/RT. Name the factor which is to be decreased to bring an increase in the rate of reaction .
Answer: Energy of activation (Ea)
Question. For a second order reaction 2A → Product, a plot t1/2 vs log a (a = initial conc.), what does the intercept represent?
Answer: t1/2 = 1/k .1/[Ao]
log t1/2 = -log K – log [Ao]
Intercept = t1/2
2- MARKS QUESTION
Question. A substance undergoes first order decomposition. The decomposition follows two parallel first order reaction as A → B + C
Find out the % distribution of B and C.
Answer: B =76.8% C= 23.2%
Percentage of formation of B = K1/K1+K2 X 100
Question. A systematic plot of ln Keq versus 1/T for a reaction has been shown below: Prove that this reaction is exothermic.
Answer: log K2 /K1 = DH/ 2.303R (1/T1 –1/T2)
log 6 /2 = DH/ 2.303R (1.5 X10-3 –2.0 X10-3)
ΔH comes negative. Hence exothermic
Question. Proposed mechanism for below given reaction :
2 NO + Br2 à 2NOBr is as follows
NO(g) + Br2(g) à NOBr2(g)
NOBr2(g) + NO(g) à 2NOBr(g)
Find out the order w.r.t. NO (g)
Answer: Rate = K [NOBr2 ][NO]
But [NOBr2]/[NO][Br2] = K
Or [NOBr2] =K[NO][Br2]
Substituting values of [NOBr2] in rate law
Rate = K’[NO]2[Br2]
Question. The rate for a reaction between the substance A and B is given by Rate= k[A]n [B]m On doubling the conc. of A and halving the conc. of B, find out the ratio of new rate to that of earlier rate of reaction .
Answer: Earlier rate R = K[A]n[B]m
Now rate R1 = K[2A]n[B/2]m
R1/R = 2n-m
Question. The following rate data was obtained for first order thermal decomposition of
SO2Cl2 at constant volume
SO2Cl2(g) à SO2(g) +Cl2(g)
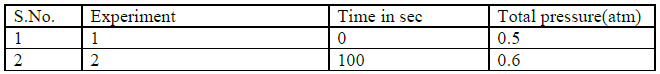
Calculate the reaction rate when total pressure is 0.56 atm.
Answer: SO2Cl2(g) → SO2(g) +Cl2(g)
T =100s 0.5 -x x x
0.5 –x +x+x = 0.60
x=0.10
K= 2.303/100 log 0.5/0.4 = 2.23 X 10-3 s-1
Similarly k can be calculated when
0.5 –x +x+x = 0.65
Question. Decomposition of NH3 (g) on surface of catalyst
2NH3 à N2 (g) + 3H2(g)
Under low pressure follows first order kinetics while at high pressure it is zero order reaction. Why?
Answer: In heterogenous catalysis molecules of NH3 are absorbed on surface. Under lower conc. the surface of catalyst is not completely occupied . When pressure is high the surface is completely occupied and further increase in pressure (conc.) does not affect the rate.
Question. In the reversible reaction
Find out the rate of disappearance of NO2
Answer: Rate of reaction = -1/2 d[NO2]/dt
= K1 [NO 2] 2 –K2 [N2O4]
therefore rate of disapperance of NO2
= - d[NO2]/dt = 2K1 [NO 2] 2 –2 K2 [N2O4]
3 - MARKS QUESTIONS
Question. For the reaction, the energy of activation is 75KJ / mol. When a catalyst is added the reaction its energy of activation is lowered to 20KJ / mol. What is the effect of catalyst on the rate of reaction at 200C.
Answer: Log K =log A-Ea/2.303RT
Log K’ =log A-Ea’/2.303RT
Log K =log A-Ea/2.303RT
Log K’/K = Ea-Ea’/2.303 RT = 9.8037
Or K’/K = 6.36 X 109
UR catalysted /UR uncatalysed =K’/K = 6.4X 10 9
Question. The gas phase decomposition of CH3OCH3 follows first order kinetics
CH3OCH3 -------> CH4 (g) + H2 (g) + CO (g)
The reaction is carried out in a constant volume container at 5000C and has t1/2 =14.5 min. Initially only dimethyl ether is present at a pressure of 0.40 atm. What is the total pressure of the system after 12 min? Assume ideal behavior.
Answer: K=0.693/t1/2
(a-x)∞ (0.40 –P)atm.
K= 2.303/12 log 0.40/0.40 –P
P calculated = 0.1745 atm
Total pressure = 0.4 –P+P+P+P =0.749 atm
Question. A heterogenous reaction is carried out at 500 K. If the same reaction is carried out in the presence of catalyst at the same rate, the temperature requires is 400 K, calculate the activation energy of the reaction if the catalyst lowers the activation barrier by 20 KJ/mol.
Answer: K=Ae-Ea/RT
Ea ,absence of catalyst = x KJ/mol
Ea, presence of catalyst = (x-20)KJ/mol
At 500K
K=Ae-x/R.500 (1)
At 400
K=Ae-(x-20)/R.400 (2)
Divide 1 by 2, we get
X=100KJ/mol
Ea = 100KJ/mol
Question. 50% of the original amount of a reactant was added to the reaction mixture after 40 min. What % of the total amount will be present after 60 min, given that half life period of the reaction is 20 min.
Answer: 40 min=2 half lives
Amount present after 40 min =a/4
Amount added = a/2
Total amount after 40 min =a/4 + a/2 = 3a/4
After 60 min from start , amount left = 3a/8
Total amount taken = a+ a/2 = 3a/2
Hence % = 3a/8 ÷ 3a/2 X 100 =25%
Question. The activation energy for the reaction :
2HI(g) H2(g) + I2(g) is 209.5KJ\mol at 581 K. Calculate the fraction of molecule of Mreactants having energy equal to or greater than activation energy.
Answer: Fraction of molecules having energy equal to or greater than activation energy
X = n/N =e –Ea/RT
Ln X = - Ea/RT
Log X = -Ea/2.303RT
= 209.5X103/2.303 X 8.314 X 581
=-18.8323
X= 1.471 X 10-19
1 Mark Questions
1. If rate law is; rate = [A]3/2 [B] -1 , determine the order.
2. A gas decomposition of AB follows the rate law; rate = K[AB]3/4. Write units of K.
3. State any one condition under which a bimolecular reaction may be kinetically of first order.
4. In some cases, it is found that a large number of colliding molecules have energy more than threshold energy, yet the reaction is slow. Why?
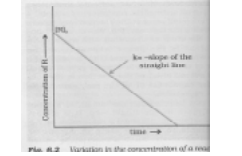
5. Variation of concentration of a reactant with time for a given reaction is shown below. What is its order of reaction?
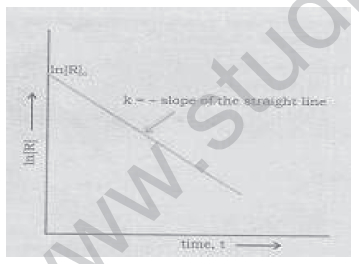
6. Variation of concentration of a reactant, ln[R] with time for a given reaction is shown below. What is its order of reaction?
2 / 3 Mark Questions
7. The kinetics of the reaction: A + 2B → Products; obeys the rate equation Rate = k [A]X [B]Y. For it, find
a) Order of the reaction
b) Apparent molecularity of reaction
c) Order of reaction when B is in large excess.
8.Following reaction takes place in one step 2NO (g) + O2 (g) → 2 NO2 (g)
How will the rate of above reaction change if the volume of the reaction vessel is diminished to one third of its original volume? Will there be any change in order of reaction with the reduced volume?
9. For the reaction
NO2 + CO → CO2 + NO
Mechanism of reaction is
a) NO2 + NO2 → NO + NO3 (slow)
b) NO3 + CO → CO2 + NO2 (fast)
How will the rate of above reaction change if the volume of the reaction vessel is diminished to one third of its original volume? Will there be any change in order of reaction with the reduced volume?
9. For the reaction
NO2 + CO → CO2 + NO
Mechanism of reaction is
a) NO2 + NO2 → NO + NO3 (slow)
b) NO3 + CO → CO2 + NO2 (fast)
Write its rate law.
10. The activation energy of a first order reaction is 30 kJ/mol at 298K. The activation energy for the same reaction in the presence of a catalyst is 24 kJ/mol at 298K. How many times the reaction rate has changed in the presence of a catalyst?
11. A reaction is carried out at two different initial concentrations of a reactant. The initial concentrations are 1mol L-1 and 2mol L-1. The half-life values obtained were20minutes and 40 minutes respectively. What is the order of reaction?
12. In the Arrhenius equation fora certain reaction, the value of A and Ea are 4× 1013 s-1 and 98.6 kJ mol-1 respectively. If the reaction is of first order; at what temperature will its half life period be ten minutes?
13. The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308K. If the pre-exponential factor for the reaction is 3.56× 109 sec-1,
calculate its rate constants at 318K and also the energy of activation.
5 Mark Questions
14. Following is a graph between reaction co-ordinate and potential energy. Explain how a catalyst influence the reaction.
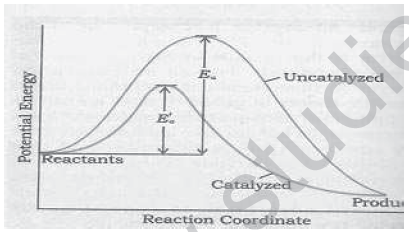
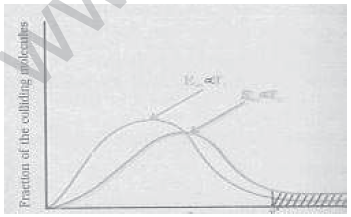
15. In a given graph, if ‘E’ is the activation energy for a given reaction, explain how temperature influences the rate of reaction.
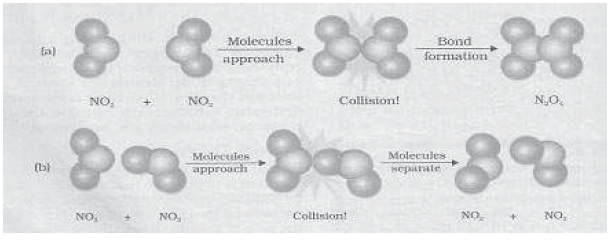
16. In the following figure, orientations of reaction molecules are shown. Explain the influence of orientation of molecules in a chemical reaction?