Read and download the CBSE Class 9 Chemistry Structure of the Atom Worksheet Set A in PDF format. We have provided exhaustive and printable Class 9 Science worksheets for Chapter 4 Structure of the Atom, designed by expert teachers. These resources align with the 2025-26 syllabus and examination patterns issued by NCERT, CBSE, and KVS, helping students master all important chapter topics.
Chapter-wise Worksheet for Class 9 Science Chapter 4 Structure of the Atom
Students of Class 9 should use this Science practice paper to check their understanding of Chapter 4 Structure of the Atom as it includes essential problems and detailed solutions. Regular self-testing with these will help you achieve higher marks in your school tests and final examinations.
Class 9 Science Chapter 4 Structure of the Atom Worksheet with Answers
Assertion & Reasoning Based MCQs
For question numbers 51-60, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Both assertion and reason are true, and reason is correct explanation of the assertion.
(b) Both assertion and reason are true, but reason is not the correct explanation of the assertion.
(c) Assertion is true, but reason is false.
(d) Assertion is false, but reason is true.
Question. Assertion : Cathode rays get deflected towards the positive plate of electric field.
Reason : Cathode rays consist of negatively charged particles known as electrons.
Answer. A
Question. Assertion : Anions are larger in size than the parent atom.
Reason : In an anion, the number of protons in the nucleus is less than the number of electrons moving around it.
Answer. A
Question. Assertion : Thomson’s atomic model is known as ‘raisin pudding’ model.
Reason : The atom is visualized as a pudding of positive charge with electrons (raisins) embedded in it.
Answer. A
Question. Assertion : The number of electrons gained, lost or shared by the atom of an element so as to complete its octet is called the valency of the element.
Reason : Elements having the same number of valence electrons in their atoms possess different chemical properties.
Answer. C
Question. Assertion : For noble gases, valency is zero.
Reason : Noble gases have 8 valence electrons.
Answer. A
Question. Assertion : The size of the nucleus is very small as compared to the size of the atom.
Reason : The electrons revolve around the nucleus of the atom.
Answer. B
Question. Assertion : Electrons moving in the same orbit will not lose or gain energy.
Reason : On jumping from higher to lower energy level, the electron will gain energy.
Answer. C
Question. Assertion : Bohr’s orbits are called stationary orbits.
Reason : Electrons remain stationary in these orbits for sometime.
Answer. C
Question. Assertion : The distribution of electrons in different orbits or shells is governed by a scheme known as Bohr-Bury scheme.
Reason : Electrons are filled in the shells in a stepwise manner in increasing order of energy of the energy shell.
Answer. B
Question. Assertion : In Rutherford’s gold foil experiment, very few a-particles are deflected back.
Reason : The size of the nucleus is very small as compared to the size of the atom
Answer. B
Exam Questions NCERT Class 9 Science Chapter 4 Structure of the Atom
Question. Give the postulates of Dalton’s atomic theory.
Answer.
(i) Every element is composed of extremely small particles called atoms.
(ii) Atoms of a given element are identical, both in mass and properties. Different chemical elements have different kinds of atoms; in particular, their atoms have different masses.
(iii) Atoms cannot be created, destroyed or transformed into atoms of other elements.
(iv) Compounds are formed when atoms of different lements combine with each other in small whole number ratios.
(v) The relative number and kinds of atoms in a given compound are constant.
Question. Give the mass numbers of A and B, What is the relation between the two species?
Answer. Mass number of A = No. of protons + No. of neutrons = 6 + 6 = 12u
Mass number of B = No. of protons + No. of neutrons = 6 + 8 = 14u
The species A and B are isotopes, as they have same atomic number but different mass number.
Question. Name the three subatomic particles of an atom.
Answer. (i) Electrons (negatively charged particles) which revolve around the nucleus.
(ii) Protons (positively charged particles) which are present in the nucleus.
(iii) Neutrons (having no charge) which are present in the nucleus.
Question. Give four characteristics of isotopes.
Answer. All isotopes of an element consist of the same number of protons inside their nuclei. Hence, they have the same atomic number.
(i) All isotopes of an element consist of different number of neutrons in their nuclei. Hence, they have different mass number.
(ii) All isotopes of an element give identical chemical reactions.
(iii) Isotopes of an element have same electronic configuration.
Question. Write the conclusions drawn by Rutherford for the following observation during his scattering experiment :
(i) Most of the alpha-particles passed straight through the gold foil.
(ii) Some alpha-particles getting deflected from their path.
(iii) Very small fraction of alpha-particles getting deflected by 180°.
Answer.
(i) Most of the space inside the atom is empty.
(ii) It indicates that the positive charge of the atom occupies a very little space.
(iii) All the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
Question. What is the difference between Rutherford’s atomic model and Thomson’s atomic model?
Answer. Rutherford proposed a model in which electrons revolve around the nucleus in well-defined orbits.
There is a positively charged centre in an atom called the nucleus. He also proposed that the size of the nucleus is very small as compared to the size of the atom and nearly all the mass of an atom is centred in the nuclei. Thomson proposed the model of an atom to be similar to a christmas pudding. The electrons are studded like currants in a positively charged sphere like Christmas pudding and the mass of the atom wassupposed to be uniformly distributed.
Question. Describe the essential properties of the atomic nucleus. Compare these properties with the properties of electron.
Answer. Nucleus is small positively charged centre located in a very small space. An electron is a very small negatively charged particle with well established charge to mass ratio. The charge on electron forms the smallest unit of charge on atomic particles.
Question. Which is much closer to the nucleus of an atom out of K and L shells?
Answer. K shell is much closer to the nucleus of an atom.
Question. The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Answer. Isobars.
Question. Name the radioisotope used for examining the circulation of blood in the body.
Answer. Na-24 is the radioisotope used for examining the circulation of blood in the body.
Question. Who discovered proton?
Answer. Goldstein discovered proton.
Question. Name one element, the nucleus of which does not have any neutron.
Answer. Hydrogen.
Question. Write the names of three elementary particles which constitute an atom.
Answer. Electron, proton and neutron.
Question. Which study led to the conclusion that atoms are not indivisible?
Answer. Study of static electricity and the condition under which electricity is conducted by different substances led to the conclusion that atoms are not indivisible.
Question. If K and L shells of an atom are full, then what would be the total number of electrons in the atom? What is the valency of this element? Name the element.
Answer. The maximum numbers of electrons that can occupy K and L shells of an atom are 2 and 8 respectively. Therefore, if K and L shells of an atom are full then the total number of electrons in the atom would be 2 + 8 = 10 electrons. So, the valency of this element is zero. The element is neon (Ne).
Question. State the charge and mass of a neutron.
Answer.Neutron has no charge and its mass is equal to that of a proton.
(i) Most of the space inside the atom is empty because most of the a -particles passed through the gold foil without getting deflected.
(ii) Very few particles are deflected from their path, indicating that positive charge of the atom occupies very little space.
(iii) A very small fraction of particles was deflected by 180°, indicating that all the positive charge and mass of the gold atom were concentrated in a small volume within the atom.
Question. If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom? And (ii) what is the charge on the atom?
Answer. (i) Atomic number = Number of protons = 8
(ii) The charge of the atom is zero, as total numbern of positive charge is equal to total number of negative charge. Number of protons = Number of electrons 8 = 8
Question. What is the limitation of J.J. Thomson’s model of an atom?
Answer. The major limitation of J.J. Thomson’s model is that it does not explain how positively charged particles are shielded from negatively charged particles, without getting neutralized.
Question. Name an element which has one electron, one proton and no neutron.
Answer. Hydrogen atom (1H1) has one electron, one proton and no neutron.
Question. Helium atom has an atomic mass of 4u and two protons in its nucleus. How many neutrons does it have?
Answer. Two.
Question. From the symbol 16S32, give :
(i) Atomic number of sulphur
(ii) Mass number of sulphur
(iii) Electronic configuration of sulphur
(iv) Which of the two elements given would be chemically more reactive? S, Ar
Answer.
(i) 16
(ii) 32
(iii) Electronic configuration : 2, 8, 6.
(iv) Element S, having atomic number 16 is chemically more reactive than element Ar of atomic number 18. It is because the outermost shell of the atom of element S has six electrons only and has to complete its octet, whereas the outermost-shell of the atom of element Ar is completely filled up, i.e., its octet is complete and thus it shows little
chemical activity.
Very Short Answer Type Question (VSA)
Question. Atomic number is defined in terms of protons and not in terms of electrons. Why?
Answer. An atom may lose or gain electrons, but the number of protons remains constant till the same atom exist.
Question. What will be the charge on an atom with mass number one and atomic number one?
Answer. The atom will not carry any charge because the atom contains one unit negative charge in the form of electron and one unit positive charge in the form of a proton.
Question. In Rutherford’s model of an atom, fast moving alpha (a)-particles were made to fall on a thin gold foil. State two properties of a-particles.
Answer. (a) Alpha particles are positively charged particles.
(b) They are doubly charged helium ions having a mass number of 4 (consists of 2 protons and 2 neutrons).
Question. What characteristic feature is seen in the configurations of chemically inactive elements?
Answer. Chemically inactive elements have 8 electrons in their valence shell except helium which has 2 electrons in its valence shell which is the maximum capacity of K shell.
Question. What would you conclude from the observation that cathode rays rotate a light paddle wheel placed in their path?
Answer. When a light paddle wheel is placed in the path of cathode rays, the blades of the paddle wheel begin to rotate.
It shows that cathode rays consist of material particles having mass and velocity.
Question. An oxide of an element Z has a formula Z2O3.
(a) How many electrons are there in the outermost shell of an atom of element Z?
(b) Write down the formula for the chloride of Z.
Answer. (a) An ion of element Z has the formula Z3+. Hence, it has 3 valence electrons.
(b) ZCl3
Question. Neutrons can be found in all atomic nuclei except in one case. Which is this atomic nucleus and what does it consist of?
Answer. In case of hydrogen atom, there is no neutron. It consists of only one proton.
Question. Find valencies of the elements having atomic numbers 10 and 15.
Answer. Atomic number = 10 Atomic number = 15
Electronic configuration Electronic configuration
= 2, 8 = 2, 8, 5
Valency = 0 Valency = 3
Question. Why are anode rays also called canal rays?
Answer. Canal rays are positively charged anode rays. The canal rays are called so because they pass through the holes or the canals in the cathode.
Short Answer Type Question
Question. (a) If the number of electrons in an ion is
Answer. (a) (i) Atomic number (Z) = No. of protons = 9
(ii) Charge on the ion = –1
Here, one electron is more than proton. So this one extra electron attains –1 charge on the ion.
(b) No. of electrons in M2+ ion = 10
Atomic number of atom M = 10 + 2 = 12
No. of protons in atom M = 12
Mass number of atom M = No. of protons + No. of neutrons
= 12 + 12 = 24
The element M with atomic number 12 is magnesium (Mg).
Question. The atom of an element has 9 protons, 9 electrons and 10 neutrons.
(a) What is the atomic number of the element?
(b) What is the mass number of the element?
(c) Name the element and give its electronic configuration.
(d) Predict the valency of the element.
Answer. (a) The atomic no. of element = No. of protons = 9
(b) The mass no. of element = No. of protons + No. of neutrons = 9 + 10 = 19
(c) The element with Z = 9 is fluorine (F). Its electronic configuration : 2, 7.
(d) The valency of fluorine is 1 and is calculated as 8 – 7 = 1.
Question. Element X has a proton number of 7. It also has seven neutrons.
(a) Deduce the number of electrons and the nucleon number of X.
(b) Represent X by writing the chemical symbol, including the proton and nucleon numbers.
Answer. p = 7, n = 7
(a) Number of electrons = Number of protons = 7
Number of nucleons = number of p + number of n
= 7 + 7 = 14
(b) 147X
Question. Justify the statement ‘atomic number of an element is equal to the number of electrons in a neutral atom only and not in anion’.
Answer. In neutral atom, No. of protons = No. of electrons
= Atomic number
An anion is formed by gain of one or more electrons by an atom. Therefore, anion contains more electrons than neutral atom or in other words number of electrons in anion is greater than atomic number (the number of protons).
Question. Give two points of differences between isotopes and isobars.
Answer. Differences between isotopes and isobars:
Isotopes Isobars
(i) These are the atoms of (i) These are the atoms of different elements.
the same element.
(ii) These have same atomic (ii) These have different atomic numbers.
number.
(iii) These have different (iii) These have same mass numbers.
mass numbers.
(iv) The chemical properties (iv) These have different chemical and physical properties.
of isotopes are similar but
their physical properties
are different.
Question. An element has two electrons in N-shell.
Identify the element.
Answer. An element can be identified with its atomic number (Z) which is equal to the number of electrons in its neutral atom.If there are 2 electrons in N shell, it means K, L and M shells are completely filled. Two electrons can be accommodated in K shell, eight in L shell and 8 in M shell since outermost orbit cannot have more than 8 electrons, 2 electrons go to N orbit.
Hence total number of electrons = 20
K L M N
2 8 8 2
The element is calcium.
Question. Justify the given statements :
(a) Most of the space in an atom is empty.
(b) The elements are identified by their atomic numbers and not by their mass numbers.
Answer. (a) This can be justified by Rutherford’s a-scattering experiment. Since most of the alpha particles could pass through the sheet, made up of atoms of gold, undeflected,
this means that they did not come across any obstruction.
Thus, most of the space in an atom is empty or hollow.
(b) In the study of the atomic structure, we have seen that the isotopes of an element have different mass numbers. In isobars, the atoms of different elements have same mass
numbers. However, the atomic numbers of no two elements can be the same. Therefore, the elements are identified by their atomic numbers and not by their mass numbers.
Short Answer Type Question
Question. (a) Electronic configuration of a neutral atom ‘X’ is 2, 8, 6. What is the electronic configuration of X2– ?
(b) What is a valence shell? How many electrons can be present in valence shell?
Answer. (a) X = 2, 8, 6
No. of electrons in neutral atom = 2 + 8 + 6 = 16
X + 2e– → X2–
No. of electrons in X2– = 16 + 2 = 18
Electronic configuration of X2– = 2, 8, 8
(b) The outermost shell of an atom is called valence shell.
The number of electrons that can be present in valence shell is 1 - 8.
Question. Atoms of elements R, S and T have 8, 9 and 11 protons respectively. Neon has 10 protons.
(a) What is the chemical formula of the compound formed between?
(i) R and T (ii) S and T
(b) What is the formula of a molecule of R?
Answer. R(p = 8), Electronic configuration = 2, 6
S(p = 9), Electronic configuration = 2, 7
T(p = 11), Electronic configuration = 2, 8, 1
(a) (i) RT2 (ii) ST
(b) R2
Question. (a) Which fact is proved by the following observation in Rutherford’s scattering experiment ‘very few alpha particles are deflected back’?
(b) How will you find the valency of nitrogen,oxygen and fluorine?
Answer. (a) This shows that in the centre of atom a very small positively charged body called nucleus is present.
(b) (i) Nitrogen has 5 electrons in valence shell, hence its valency is 8 – 5 = 3.
(ii) Oxygen has 6 electrons in valence shell, hence its valency is 8 – 6 = 2.
(iii) Fluorine has 7 electrons in valence shell, hence its valency is 8 – 7 = 1.
Question. (a) Explain, why 32He and 31H are not considered isotopes.
(b) What are octet and duplet rules? How do elements attain octet?
Answer.
(a) 3/2He and 3/1H are not considered as isotopes because they have different atomic numbers and are different elements.
(b) Octet rule was proposed by G.N.Lewis. According to this rule “The atom of an element combines with another atom to have eight electrons in its outermost shell”. An atom having 8 electrons in its outermost shell is least reactive or most stable. If there is only one shell, then stability is attained by having 2 electrons in the shell and this is called duplet rule.
Element attains octet in the following ways :
(i) by losing or gaining electrons.
(ii) by sharing electrons with other atoms.
Question. (a) On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
(b) What are canal rays?
Answer. (a) According to Thomson’s model of an atom :
(i) An atom consists of a positively charged sphere and the electrons are embedded like the seeds in a watermelon.
(ii) The negative and positive charges are equal in magnitude.
So, the atom as a whole is electrically neutral.
(b) The beam of rays which travel in a direction away from anode towards cathode when a gas taken in a discharge tube is subjected to the action of high voltage under low pressure are known as canal rays. It is also called anode rays. It was discovered by E. Goldstein in 1886.
Question. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer. (a) F : Because it was not proposed by J.J. Thomson.
(b) F : Because neutron is an independent sub-atomic particle.
(c) T : Because it is a fact known from experiments.
(d) F : Because tincture iodine is a solution of ordinary iodine in alcohol.
Question. (a) Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
(b) Write the distribution of electrons in carbon and sodium atoms.
Answer.
(a) Mass number of helium = 4
Number of protons = 2
Number of neutrons (n)
= Mass number (A) – No. of protons (p)
= 4 – 2 = 2
Thus, no. of neutrons = 2
(b) Atomic number of carbon = 6
Hence first shell (K-shell) have 2 electrons and the remaining 4 electrons will be present in the second shell, i.e. L-shell.
Thus the distribution will be
K L
2 4
Atomic number of sodium = 11. Hence, first shell (K-shell) will have 2 electrons and second shell (L-shell) will have 8 electrons and third shell (M-shell) will have 1 electron. Thus, the distribution will be
K L M
2 8 1
Question. Summarise the rules for writing the distribution of electrons in various shells for the first eighteen elements.
Answer.
The distribution of elements in different orbits is governed by a scheme called Bohr-Bury scheme. There are following rules :
(i) The maximum number of electrons present in any shell is given by the formula 2n2. Where n = no. of orbit.
(ii) The maximum number of electrons that can be accommodated in the outermost shell is 8.
(iii) Electrons in an atom do not occupy a new shell unless all the inner shells are completely filled.
Question. (a) What is the number of valence electrons in the atom of an element A having atomic number 20? Name the valence shell of this atom.
(b) The atom of an element has 9 protons, 9 electrons and 10 neutrons.
(i) What is the atomic number of the element?
(ii) What is the mass number of the element?
(iii) Name the element and give its electronic configuration.
(iv) Predict the valency of the element.
Answer.
(a) The electronic configuration of element A is
K L M N
2 8 8 2
Therefore, the N shell is the outermost shell or the valence shell. Number of valence electrons
= number of electrons in the outermost shell
= 2.
(b) (i) The atomic no. of element = No. of protons = 9
(ii) The mass no. of element
= No. of protons + No. of neutrons
= 9 + 10 = 19
(iii) The element with Z = 9 is fluorine (F).
Its electronic configuration : 2, 7.
(iv) The valency of fluorine is 1 and is calculated as 8 – 7 = 1.
Question. Use the information to answer the following questions :
Element P Q R S T U V
Proton number 7 8 10 12 15 18 19
(a) Which of these elements have only four filled electron shells?
(b) Which of these elements have a complete outermost shell?
(c) Which of these elements have 5 valence electrons?
(d) Which of these elements have 6 valence electrons?
(e) Which of these elements have 2 valence electrons?
(f) Write the valencies of each of the elements.
Answer.
(a) V(2, 8, 8, 1) (b) R(2, 8) and U(2, 8, 8)
(c) P(2, 5) and T(2, 8, 5) (d) Q(2, 6)
(e) S(2, 8, 2)
(f) P(3), Q(2), R(0), S(2), T(3), U(0), V(1)
Question. (a) Explain the formation of a cation. Give its main characteristics.
(b) What is electronic configuration of Al3+?
Answer.
(a)A cation is formed when an atom loses one or more than one electrons from valence shell.
M → M+ + e–
For example,
Na → Na+ + 1e–
Mg → Mg2+ + 2e–
Characteristics of cations :
(i) Cations are positively charged.
(ii) Cations are formed when an atom loses electrons from its valence shell to attain octet.
(iii) Cations are smaller in size than parent atom.
(iv) The charge acquired by a cation is equal to the number of electrons lost by the valence shell.
(b)Atomic number of aluminium is 13 and its electronic configuration is 2, 8, 3. Al3+ is formed by removing 3 electrons from aluminium atom.
Al – 3e– → Al3+
Hence, electronic configuration of Al3+ is 2, 8.
Question. How will you find the valency of chlorine, sulphur and magnesium?
Answer. Valency of an atom is the number of electrons gained,lost or shared so as to complete the octet of electrons in the valence shell.
Valency of chlorine: It has electronic configuration = 2, 8, 7
Thus, one electron is gained to complete its octet and so its valency is 1.
Valency of sulphur: It has electronic configuration = 2, 8, 6
Thus, two electrons are gained to complete its octet and hence its valency = 2
Valency of magnesium : It has electronic configuration = 2, 8, 2
Thus, it can lose two electrons to attain octet and hence its valency = 2
Long Answer Type Question
Question. Observe the given figure and answer the questions that follow :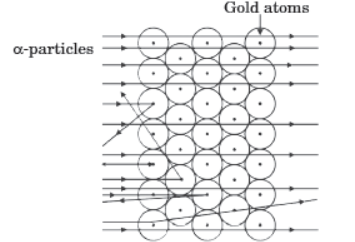
(a) Which experiment is represented by the given figure?
(b) List three observations of this experiment.
(c) State conclusions drawn from each observation of this experiment.
(d) Write the features of the nuclear model of an atom.
(e) What were the drawbacks of this model of an atom?
Answer. (a) Rutherford’s a-particle scattering experiment
(b) Rutherford observed that :
(i) Most of the a-particles (nearly 99%) passed through the gold foil undeflected.
(ii) Some of the a-particles were deflected by small angles.
(iii) A very few a-particles (1 in 12,000) were either deflected by very large angles or were actually reflected back along their path.
(c) Rutherford explained his observation as follows :
(i) Since most of the a-particles passed through the foil undeflected, it indicates that the most of the space in an atom is empty.
(ii) a-Particles being positively charged and having considerable mass, could be deflected only by some heavy, positively charged centre. The small angle of deflection of a-particles indicated the presence of a heavy positive centre in the atom. Rutherford named this positive centre as nucleus.
(iii) a-Particles which make head-on collision with heavy positive centre are deflected through large angles. Since
Question. Give reasons for the following :
(a) Isotopes of an element are chemically similar.
(b) An atom is electrically neutral.
(c) Noble gases show least reactivity.
(d) Nucleus of an atom is heavy and positively charged.
(e) Ions are more stable than atoms.
Answer. (a) Isotopes of an element have same atomic number as well as electronic configuration. Since the chemical properties of elements are related to their electronic configuration, i.e., the elements with similar configuration will have similar properties. Thus, the isotopes of an element are chemically similar.
(b) In an atom, the number of protons in the nucleus is equal to the number of electrons in the extra-nuclear portion. Since each proton and each electron has the same charge but with opposite magnitude, the atom is electrically neutral.
(c) The atoms of noble gas elements have complete outermost shells. Hence, they are least reactive.
(d) Nucleus of an atom is made up of protons which are positively charged and neutrons that are neutral. Hence net charge on nucleus is positive. The total mass of neutron and
proton makes it heavy.
(e) When an atom changes into an ion (cation or anion) the valence shell of the ion has a complete octet or duplet which makes ions more stable than atoms.
Question. The table shows the numbers of electrons, neutrons and protons in some atoms and ions of elements.
(The letters used in the table are not the chemical symbols of the elements.)
Answer.
(a) V
(b) U
(c) T and W
(d) S
(e) S
| CBSE Class 9 Chemistry Matter In Our Surrounding Worksheet Set A |
| CBSE Class 9 Chemistry Matter In Our Surrounding Worksheet Set B |
| CBSE Class 9 Chemistry Is Matter Around Us Pure Worksheet Set A |
| CBSE Class 9 Chemistry Is Matter Around Us Pure Worksheet Set B |
| CBSE Class 9 Physics Motion Worksheet Set A |
| CBSE Class 9 Physics Motion Worksheet Set B |
| CBSE Class 9 Physics Gravitation Worksheet Set A |
| CBSE Class 9 Physics Gravitation Worksheet Set B |
| CBSE Class 9 Physics Work And Energy Worksheet Set A |
| CBSE Class 9 Physics Work And Energy Worksheet Set B |
| CBSE Class 9 Physics Work And Energy Worksheet Set C |
| CBSE Class 9 Physics Sound Worksheet Set A |
| CBSE Class 9 Physics Sound Worksheet Set B |
| CBSE Class 9 Physics Sound Worksheet Set C |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set A |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set B |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set C |
| CBSE Class 9 Biology Natural Resources Worksheet Set A |
| CBSE Class 9 Biology Natural Resources Worksheet Set B |
| CBSE Class 9 Biology Natural Resources Worksheet Set C |
Important Practice Resources for Class 9 Science
CBSE Science Class 9 Chapter 4 Structure of the Atom Worksheet
Students can use the practice questions and answers provided above for Chapter 4 Structure of the Atom to prepare for their upcoming school tests. This resource is designed by expert teachers as per the latest 2026 syllabus released by CBSE for Class 9. We suggest that Class 9 students solve these questions daily for a strong foundation in Science.
Chapter 4 Structure of the Atom Solutions & NCERT Alignment
Our expert teachers have referred to the latest NCERT book for Class 9 Science to create these exercises. After solving the questions you should compare your answers with our detailed solutions as they have been designed by expert teachers. You will understand the correct way to write answers for the CBSE exams. You can also see above MCQ questions for Science to cover every important topic in the chapter.
Class 9 Exam Preparation Strategy
Regular practice of this Class 9 Science study material helps you to be familiar with the most regularly asked exam topics. If you find any topic in Chapter 4 Structure of the Atom difficult then you can refer to our NCERT solutions for Class 9 Science. All revision sheets and printable assignments on studiestoday.com are free and updated to help students get better scores in their school examinations.
You can download the latest chapter-wise printable worksheets for Class 9 Science Chapter Chapter 4 Structure of the Atom for free from StudiesToday.com. These have been made as per the latest CBSE curriculum for this academic year.
Yes, Class 9 Science worksheets for Chapter Chapter 4 Structure of the Atom focus on activity-based learning and also competency-style questions. This helps students to apply theoretical knowledge to practical scenarios.
Yes, we have provided solved worksheets for Class 9 Science Chapter Chapter 4 Structure of the Atom to help students verify their answers instantly.
Yes, our Class 9 Science test sheets are mobile-friendly PDFs and can be printed by teachers for classroom.
For Chapter Chapter 4 Structure of the Atom, regular practice with our worksheets will improve question-handling speed and help students understand all technical terms and diagrams.

