THE SOLID STATE
1. What is the number of atoms in a unit cell of simple cubic, BCC and FCC crystals?
Number of atoms in primitive cube ( Rank) = 1
Number of atoms in BCC (Rank) = 2
Number of atoms in FCC (Rank) = 4
2. State a feature to distinguish
(i) Metallic solid from ionic solid
(ii) Covalent solid from molecular solid
Constituent particles in metallic solids are positive metal ions (kernels) and mobile electrons. Constituent particles in ionic solids are positive ions and negative ions. Constituent particles in covalent solids are atoms which are connected by covalent bond. Constituent particles in molecular solids are molecules which are held by van der waal’s force of attraction.
3. What type of alignment in crystals makes them ferromagnetic, antiferromagnetic and ferrimagnetic?
(i) Ferromagnetism arises due to spontaneous alignment of magnetic moments of ions or atoms in the same direction.
(ii) Antiferromagnetism arises due to alignment of magnetic moments in opposite direction resulting in zero magnetic moment.
(iii) Ferrimagnetism arises due to alignment of magnetic moments in parallel and antiparallel directions in unequal numbers resulting in a net magnetic moment.
4. How would you account for the following?
(i) Frenkel defects are not found in ionic solids of nearly equal sizes of cations and anions.
(ii) Schottky defects lower the density of a crystalline solid.
(iii) Impurity doped silicon is a semiconductor.
(i) Larger cation can not slip and occupy the interstitial gap.
(ii) Since ions are missing from their respective lattice positions leaving behind holes, Schottky defects lower the density of a crystalline solid.
(iii) When silicon is doped with group 15 impurities, they use four out of five electrons for covalent bond formation while the fifth electron is extra and conducts electricity. These are called n-type semiconductors. When silicon is doped with group 13 impurities, they form three covalent bonds and electron holes. These are called p-type semiconductors.
5. Explain the following with suitable example.
(i) Frenkel defect
(ii) F-centres
(iii) Paramagnetism
(i) This defect is created when an ion leaves its correct lattice site and occupies an interstitial site. It creates vacancy defect at its original site and interstitial defect at its new location. This defect does not change the density of solid.
(ii) In non-stoichiometric ionic solids the anion vacancies or holes occupied by electrons are called F-centres. These are responsible for the colour of the compound.
(iii) Paramagnetism is the property which arises due to the presence of one or more unpaired electrons. Paramagnetic substances are those substances which are weakly attracted by a magnetic field.
6. Iron has a body centred cubic unit cell with cell edge of 286.65pm. The density of iron is 7.87gcm-3. Use this information to calculate Avogadro’s number. (Atomic mass of Fe = 56 g mol-1)
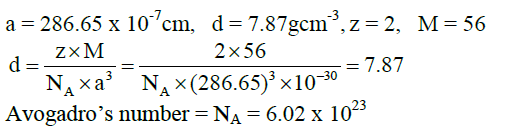
Solutions
7. Non-ideal solutions exhibit either positive or negative deviations from Raoult’s law. What are these deviations and how are they caused?
Positive Deviation:
If the observed vapour pressure of solution is higher than that predicted by Raoult’s law, then the solution exhibits positive deviation. The solvent-solute interaction is weaker than solvent-solvent and solute-solute interactions.
Properties of solutions showing positive deviation:
(i) Since the solvent-solute interactions are weaker, the vapour pressure of solution is higher than that predicted by Raoult’s law pA > pA° χA and pB > pB° χB ;
(ii) Due to decrease in attractive forces, the dissolution process is endothermic.
ΔHmix>0.
(iii) Due to decrease in attractive forces, the molecules will be loosely held. Hence there will be increase in volume on mixing. ΔVmix > 0. Mixture of ethanol and acetone shows positive deviation from ideal behaviour.
Negative Deviation:
If the observed vapour pressure of solution is lower than that predicted by Raoult’s law, then the solution exhibits negative deviation. The solvent-solute interactions are stronger than the solvent-solvent and solute-solute interactions. Properties of solutions showing negative deviation :
(i) Since the solvent-solute interactions are stronger, the vapour pressure of solution is lower than that predicted by Raoult’s law pA < pA° χA and pB < pB° χB ;
(ii) Due to increase in attractive forces, the dissolution process is exothermic. ΔHmix < 0.
(iii) Due to increase in attractive forces, the molecules will be held more tightly. Hence there will be decrease in volume on mixing. ΔVmix < 0. A mixture of chloroform
8. Define the term osmosis and osmotic pressure. Osmotic pressure method is more advantageous in determining molar mass non-volatile solutes over other colligative properties. Why?
Osmosis is defined as the process by which solvent particles move from a solution of lower concentration to a solution of higher concentration through a semi- permeable membrane.
Osmotic pressure is defined as the excess pressure that must be applied to the concentrated solution side to prevent the movement of solvent through semipermeable membrane.
Osmotic pressure method is more advantageous because
o It can be measured at room temperature.
o Other colligative properties are too small to be measured but osmotic pressure will be accurate and appreciable for such substances. It can be measured more accurately over other colligative properties.
9. 100mg of protein is dissolved in just enough water to make a 10mL solution. If this solution has an osmotic pressure of 13.3mm Hg at 250C, what is the molar mass of the protein? (Given: R = 0.0821L atm K-1 mol-1,
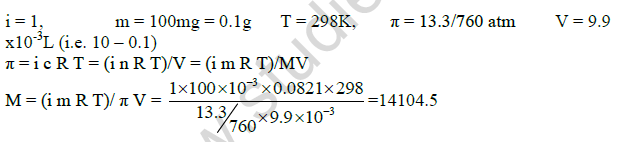
10. What do you mean by abnormal molar mass? Explain the factors The experimental molar mass determined by colligative properties when there is dissociation or association of solute particles is known as abnormal molar mass.
Dissociation of solute:
If there is disociation of solute, the number of particles would increase and the colligative proprties will be higher than expected. Since the colligative properties are inversely proportional to molar mass, the experimental molar mass will be lower than the correct value.
Association of solute:
If there is association of solute, the number of particles would decrease and the colligative proprties will be lower than expected. Since the colligative properties are inversely proportional to molar mass, the experimental molar mass will be higher than the correct value.
Please click on below link to download CBSE Class 12 Chemistry All Chapters Solved Worksheet

