Question 9. An acid produces .......... ions in water.
(a) Hydrogen (b) Helium
(c) OH− (d) None of these
Question 10. When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction is
SO2 + 2H2S →2H2O + 3S
Here hydrogen sulphide is acting as:
(a) an oxidising agent (b) a reducing agent
(c) a dehydrating agent (d) a catalyst
Question 11. Which of the following statements is true about Trachea in a respiratory system?
(a) It functions as passages of air to each alveolus
(b) It functions for sound production
(c) It Acts as passage of air to bronchi
(d) It Lowers the surface tension
Question 12. The function of sphincter muscle is to
(a) Regulate digestive process
(b) Improves digestion
(c) Release digestive juices
(d) Regulate exit of food
Question 13. Autotrophic organism converts of carbon dioxide and water into carbohydrates in the presence of
(a) Sunlight and carbon dioxide
(b) Sunlight and chlorophyll
(c) Carbon dioxide and Nitrogen
(d) Chlorophyll and carbon dioxide
Question 14. Each kidney has large numbers of filtration units which is called ___
(a) Nephrons (b) Glomerulus
(c) Renal vein (d) None of the above
Question 15. The effect of root pressure in transport of water is more important at
(a) day time (b) night time
(c) both (a) and (b) (d) none of these
Question 16. The biological process involved in the removal of these harmful metabolic wastes from the body is called
(a) Photosynthesis (b) Respiration
(c) Excretion (d) Translocation
Question 17. A ray of light is refracted as per the following diagram.
Which of the following medium is optically denser?
(a) Medium A
(b) Medium B
(c) Cannot be identify
(d) Both medium are denser
Question 18. The radius of curvature of concave mirror is 24 cm.
Then, the focal length will be
(a) −12 cm (b) 6 cm
(c) −24 cm (d) −6 cm
Question 19. The correct order of refractive index of various materials is :
(a) Diamond > Ice > Alcohol > Rock salt
(b) Ice > Diamond > Rock salt > Alcohol
(c) Diamond > Rock salt > Alcohol > Ice
(d) Rock salt > Alcohol > Ice > Diamond
Question 20. A full length of a distant tall building can definitely be seen by using
(a) a concave mirror
(b) a convex mirror
(c) a plane mirror
(d) both concave as well as plane mirror
Question 21. In torches, search light and headlights of vehicles the bulb is placed
(a) Between the pole and focus of the reflector
(b) Very near to the focus of the reflector
(c) Between the focus and centre of curvature of the reflector
(d) At the centre of curvature of the reflector
Question 22. A point source of light is placed in front of a plane mirror, then :
(a) all the reflected rays will meet at a point when produced backward
(b) only the reflected rays close to the normal will meet at a point when produced backward
(c) only the reflected rays making a small angle with the mirror will meet at a point when produced backward
(d) light of different colours will make different images
Question 23. A plane mirror reflects a beam of light to form a real image. The incident beam is :
(a) parallel
(b) convergent
(c) divergent
(d) any one of the above
Question 24. Advanced sunrise and delayed sunset are explained on the basis of
(a) Tyndall effect
(b) scattering of light
(c) dispersion of light
(d) atmospheric refraction
Question 25. Which of the following statement is incorrect about acids?
(a) they change the colour of red litmus to blue
(b) they have sour taste
(c) they may change the colour of indicator
(d) they changes the colour or blue litmus to red
Question 26. The hydroxyl ion concentration of a solution is 1.0 × 10−9M. The pH of the solution is:
(a) 4 (b) 5
(c) 6 (d) 7
Question 27. Generally metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
(a) H2SO4 (b) HCl
(c) HNO3 (d) All of these
Question 28. Which metals react with cold water?
(a) Iron, calcium, magnesium
(b) Iron, sodium, magnesium
(c) Sodium, calcium, potassium
(d) Silver, sodium, magnesium
Question 29. Aqueous solution of copper sulphate reacts with aqueous ammonium hydroxide solution to give.
(a) green precipitate (b) brown precipitate
(c) pale blue precipitate (d) white precipitate
Question 30. Two elements X and Y on burning in air give corresponding oxides. Oxides of both X and Y are soluble in water. The aqueous solution of oxide of X is alkaline and reacts with aqueous solution of oxide of Y to give another compound. Identify X and Y
(a) X and Y both are metals
(b) X and Y are non-metals
(c) X is metal and Y is non-metal
(d) X is non-metal and Y is metal
Question 31. Assertion : HCl produces hydronium ions (H3O )+ and chloride ions (Cl−) in aqueous solution.
Reason : In presence of water, base give H+ ions.
(a) Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) Both Assertion and Reason are true but reason is not the correct explanation of Assertion.
(c) Assertion is true but Reason is false.
(d) Assertion is false but Reason is true.
Question 32. Assertion : Chips manufacturers usually flush bags of chips with oxygen gas.
Reason : It adds taste to chips.
(a) Both Assertion and Reason are True and Reason
is the correct explanation of the Assertion.
(b) Both Assertion and Reason are True but Reason
is not the Correct explanation of the Assertion.
(c) Assertion is True but the Reason is False.
(d) Both Assertion and Reason are False.
Question 33. Assertion : In plants, water is transported through phloem.
Reason : It is because sieve tubes are absent in phloem.
(a) Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) Both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
(c) Assertion is true but Reason is false.
(d) Both Assertion and Reason are false.
Question 34. Assertion : In case of rainbow, light at the inner surface of the water drop gets internally reflected.
Reason : The angle between the refracted ray and normal to the drop surface is greater than the critical angle.
(a) Both Assertion and Reason are true and Reason
is the correct explanation of Assertion.
(b) Both Assertion and Reason are true but Reason is
not the correct explanation of Assertion.
(c) Assertion is true but Reason is false.
(d) Assertion is false but Reason is true.
Question 35. Which of the following is acidic in nature?
(a) Lime juice (b) Human blood
(c) Lime water (d) Antacid
Question 36. A reaction in which a single reactant breaks down to form two or more products is known as decomposition reaction. Decomposition reaction is just the opposite of combination reaction. The decomposition reaction takes place only when the energy in the form of heat, electricity or light is supplied.
Example: Ferrous sulphate crystals on heating in a dry boiling tube gives the following reaction:
Which of the following gas has a smell of burning sulphur?
(a) Sulphur oxide
(b) Sulphur dioxide
(c) Sulphur chloride
(d) None of these
Question 37. The only reptile having 4 chambered heart is:
(a) Snake (b) Turtle
(c) Lizard (d) Crocodile
Question 38. The excretory system of human beings includes?
(a) A pair of kidneys
(b) A pair of ureters
(c) A urinary bladder and a urethra
(d) All of the above
Question 39. Convergence of concave mirror can be decreased by dipping in
(a) Water (b) Oil
(c) Both (d) None of these
Question 40. Which of the following ray diagrams is correct for the ray of light incident on a concave mirror as shown in Figure?
Question 41. Trans location is the process in which plants deliver:
(a) minerals from leaves to other parts of the plant
(b) plant growth hormones from leaves to other parts of the plant
(c) water and organic substance from leaves to other parts of the plant
(d) all of the above
Question 42. Normally, in a healthy adult, the initial filtrate in the kidneys is about :
(a) 100 L/day
(b) 150 L/day
(c) 180 L/day
(d) 200 L/day
Question 43. A thin layer of water is transparent but a very thick layer of water is:
(a) translucent
(b) opaque
(c) most transparent
(d) none of these
Question 44. Which of the following correctly represents graphical relation between angle of incidence (i) and angle of reflection (r)?
Question 45. A ray of light falls normally on the surface of a transparent glass slab. The angle of emergence is-
(a) 0°
(b) 90°
(c) 45°
(d) 70°
Question 46. Two thin lenses of power +3.5 D and −2.5 D are placed in contact. The power of the lens combination is-
(a) +1D
(b) +1.5 D
(c) +2.5 D
(d) +2 D
Question 47. Parallel rays from the top of a distant object, incident on a concave mirror form an image on the screen. The diagram correctly showing the image of the object on the screen in figure is:
Answer : C
Question 48. Silver articles become black on prolonged exposure to air. This is due to the formation of
(a) Ag3N (b) Ag2O
(c) Ag2S (d) Ag2S and Ag3N
Answer : C
Section C
Case Based Questions: (49-52)
A student takes the there solutions P,Q and R and make the reaction of all these solution with phenolphthalein indicator and methyl orange indicator. He get the following result:
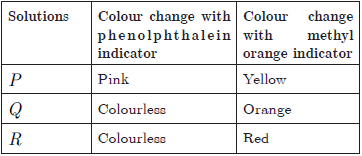
Question 49. The acidic solution is
(a) P (b) Q
(c) R (d) None of these
Question 50. The increasing of pH of solution P, Q and R is
(a) P <Q < R
(b) R < P < Q
(c) R < Q < P
(d) Q < R < P
Question 51. Solutions P and Q could be
(a) HCl and NaOH
(b) NaOH and NaCl
(c) CH3COOH and CH3COONa
(d) HCl and Na2CO3
Question 52. When solution P added to the China rose indicator, the colour of the solution P changes to
(a) Green (b) Dark red
(c) Pink (d) Colourless
Case Based Questions: (53-56)
The organs of our excretory system help in releasing waste from our body. If these wastes are not removed, we may fall sick. Hence, wastes built up from cell activities and digestion need to be removed. The excretory system of humans consists of a pair of kidneys, pair of ureter, a urinary bladder and urethra.
Kidneys are located in abdomen, one on other side of the backbone. Urine produced in kidneys passes through the ureter into the urinary bladder where it gets stored for sometime and then is released through urethra.
Each kidney is made up of one million nephrons and each nephron consists of a cup-shaped upper end called Bowman’s capsule containing a bunch of capillaries called glomerulus.
The Bowman’s capsule leads to proximal convoluted tubule, loop of Henle and distal convoluted tubule which joins the collecting duct.
Question 53. Which among the following is the least toxic form of excretory product?
(a) Urea (b) Uric acid
(c) Ammonia (d) CO2
Question 54. An outline of principal events of urination is given below in a random manner.
I. Stretch receptors on the wall of the urinary bladder send signals to CNS.
II. Bladder fills with urine and become distended.
III. Micturition.
IV. CNS passes on motor messages to initiate contraction of smooth muscle of bladder and simultaneous relaxation of urethral sphincter.
The correct sequence of events is
(a) I →II → III →IV
(b) IV → III → II →I
(c) II → I → IV → III
(d) III → II → I → IV
Question 55. A person with no/less food and beverage intake, will have .......... in urine.
(a) little glucose (b) less urea
(c) excess urea (d) little fat
Question 56. Glomerular filtrate is first collected by
(a) distal convoluted tubule
(b) proximal convoluted tubule
(c) Bowman’s capsule
(d) Loop of Henle
Case Based Questions: (57-60)
The hotter air is lighter (less dense) than the cooler air above it and has a refractive index slightly less than that of the cooler air. Since the physical condition of the refracting medium (air) are not stationary, therefore, the light goes from rarer medium to denser medium in atmosphere. This phenomenon is called atmospheric refraction.
The twinkling of stars and advanced sunrise and delayed sunset are common examples of atmospheric refraction.
Question 57. Stars appear to twinkle because of
(a) movement of air
(b) atmospheric refraction
(c) both (a) and (b)
(d) none of these
Question 58. Which of the following is not caused because of atmospheric refraction?
(a) Apparent image of Sun is formed closer to the Earth.
(b) Dawn or dusk are formed
(c) Sun can be seen 2 minutes before actual sunrise and 2 minutes after actual sunset.
(d) Clouds look white
Question 59. During sunset or sunrise the Sun appears reddish because
(a) due to longer passage in atmosphere, even red light in the sunlight scatters
(b) Sun produces red light at this time
(c) at this time Sun is not very hot
(d) none of these
Question 60. When sunlight enters the atmosphere the colours which scatter first are
(a) only red
(b) red, orange and yellow
(c) blue and green
(d) violet, indigo and blue

